Abstract
Advanced macrolides, such as azithromycin (AZM) or clarithromycin (CLM), are antibiotics with immunomodulatory properties. Here we have sought to evaluate their in vitro influence on the activation of CD4+ T-cells. Isolated CD4+ T-cells were stimulated with agonistic anti-CD3/anti-CD28 monoclonal antibodies in the presence of 0.6 mg/L, 2.5 mg/L, 10 mg/L or 40 mg/L AZM or CLM. Cell proliferation, cytokine level in supernatants and cell viability was assessed. Intracellular signaling pathways were evaluated using reporter cell lines, FACS analysis, immunoblotting and in vitro kinase assays. AZM inhibited cell proliferation rate and cytokine secretion of CD4+ T-cells in a dose-dependent manner. Similarly, high concentrations of CLM (40 mg/L) also suppressed these T-cell functions. Analysis of molecular signaling pathways revealed that exposure to AZM reduced the phosphorylation of the S6 ribosomal protein, a downstream target of mTOR. This effect was also observed at 40 mg/L CLM. In vitro kinase studies using recombinant mTOR showed that AZM inhibited mTOR activity. In contrast to rapamycin, this inhibition was independent of FKBP12. We show for the first time that AZM and to a lesser extent CLM act as immunosuppressive agents on CD4+ T-cells by inhibiting mTOR activity. Our results might have implications for the clinical use of macrolides.
Similar content being viewed by others
Introduction
Macrolides are a group of antimicrobial substances with well-described immunomodulatory properties1,2. They inhibit bacterial protein synthesis by reversibly binding to the prokaryotic 50S ribosomal subunit3, whereas effects on eukaryotic ribosomes are not described. Due to their beneficial pharmacokinetics, advanced macrolides, including azithromycin (AZM) and clarithromycin (CLM), are widely used to medicate respiration tract infections, sexually transmitted diseases and Helicobacter pylori-associated peptic ulcer diseases4. Due to a high degree of cellular penetration, AZM and CLM reach 10 to 100 fold higher concentrations in body tissues and enrich in leukocytes at more than 100 fold higher concentrations compared serum levels5,6,7. The penetration efficacy depends on environmental factors, such as pH, extracellular calcium concentrations, temperature and inflammatory stimuli, as well as on the cell type8,9,10. In contrast to CLM and other macrolides, AZM has unique characteristics, being an azalide derivate which does not inhibit the human CYP3A4 oxidases1.
Particularly, in patients suffering from lung diseases and chronic diseases with an auto-immune component, beneficial therapeutic effects of macrolide treatment have been observed. Clinical efficacy of AZM and CLM has been shown for the prevention of exacerbation in patients with chronic obstructive pulmonary disease (COPD)11,12, cystic fibrosis13,14 and non-cystic fibrosis bronchiectasis15. Similarly, in patients suffering from diffuse panbronchiolitis16, bronchiolitis obliterans syndrome after lung transplantation17,18 and patients with asthma19,20,21, clinical improvements have been demonstrated following administration of AZM or CLM.
Obviously, immunomodulatory effects might also occur in patients without the need of any immunotherapy, e.g. patients during the immunodepressed phase of a severe sepsis22 or HIV positive patients with a low CD4+ count. Especially, the latter group of patients is of high relevance, since AZM and CLM are the first line prophylaxis against atypical mycobacterial infections in HIV positive patients with less than 50 CD4+ T-cells per μl peripheral blood23.
Thus, in order to fully harness the immunomodulatory potential of macrolides, the assessment of their effects on individual immune cell subsets is necessary. To date, research has mainly focused on the innate immune system, especially characterizing macrolide mediated modulation of monocytes24,25,26,27. At the cytokine expression level, alterations of various pro-inflammatory cytokines, such as interleukin (IL)-1 beta, IL-8, IL-17 or tumor necrosis factor (TNF) - alpha have been described28,29. Several candidates are discussed as the underlying molecular targets responsible for these effects, including cellular c-jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK) and activator protein-1 (AP-1)24,25,28.
Yet, only few studies about the impact of AZM or CLM on adaptive immune cells were conducted. In these studies, both AZM and CLM were identified as inducers of apoptosis in lymphocytes at concentrations at or above 100 mg/L30,31,32. Mechanistically, these effects were dependent on the Fas-FasL pathway and down-regulation of the anti-apoptotic protein Bcl-xL. These studies provide first indications that macrolides might also influence lymphocyte responses. However, the exact molecular targets as well as the influence of AZM and CLM on distinct lymphocyte subsets have not been fully characterized so far.
Among a plethora of intracellular signaling cascades, only a few are critical for T-cell activation. These pathways also constitute targets of immunosuppressive drugs. The mammalian target of rapamycin (mTOR) pathway represents one of these critical factors. The mTOR, a serine/threonine protein kinase, mainly acts as a sensor for the nutrient state of T-cells, but is also regulated by the CD28 co-stimulatory receptor33. The phosphoinositide 3-kinase (PI3-K) acts as an important signaling module upstream of mTOR34. Rapamycin (RAPA), a macrolide without bactericidal activity, inhibits mTOR function via complex formation with the FK506 binding protein 12 (FKBP12). This inhibition is sufficient to completely abrogate T-cell activation including proliferation and cytokine secretion. Due to this potency, RAPA is routinely used in clinical application as an immunosuppressive drug for the prevention and therapy of transplant rejection35. However, reports also show that the usage of RAPA can be accompanied by severe side effects including lymphocytic alveolitis, interstitial pneumonitis and severe forms of glomerulonephritis36.
In consideration of its clinical relevance, investigations on the effects of macrolides on well-defined populations of adaptive immune cells are highly warranted. Consequently, the aim of this study was to assess effects of the AZM and CLM on CD4+ T-cell effector functions. For that purpose, the effect of AZM and CLM on CD4+ T-cell activation and on intracellular signaling factors including ERK, p38 and mTOR were studied.
Results
Suppression of CD4+ T-cells proliferation following activation
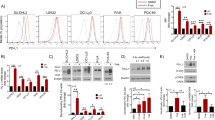
Highly purified CD4+ T-cells were activated in the presence of titrated levels of AZM (Zithromax®), CLM (Klacid®), or antibiotic-free medium. In thymidine incorporation assays, cell proliferation levels of CD4+ T-cells were significantly reduced in cells exposed to indicated macrolides (p < 0.001, see: Figure 1A; for absolute values from one representative donor see Supplementary Figure 1). A slight reduction of cellular proliferation was found already at 0.6 mg/L AZM, which did not reach statistical significance (p = 0.21). At a fourfold higher concentration (2.5 mg/L) a significant reduction of cellular proliferation was observed in T-cells exposed to AZM (p = 0.001), but not following exposure to CLM (p > 0.999). A concentration of 10 mg/L AZM reduced T-cell proliferation to a median proliferation rate of 0.45 (p < 0.001). In contrast to AZM, the presence of CLM at this concentration had no significant influence on the proliferation rate (median: 0.72, p = 0.066). At 40 mg/L AZM, T-cell proliferation was strongly reduced (median: 0.10, p < 0.001), whereas exposure to 40 mg/L CLM suppressed the median proliferation to 0.54 (p = 0.002). Generally, the magnitude of the suppressive effect of AZM was significantly higher than the effect of CLM (0.6 mg/L: p = 0.023, 2.5 mg/L: p = 0.008, 10 mg/L p = 0.008, 40 mg/L: p = 0.014). Results did not depend on the used drug formulation (i.v. formulation versus highly purified substance, data not shown). Each increase of AZM dose led to a significant reduction of the T-cell proliferation (p values: ≤0.001), indicating a dose-dependency in this suppression. For confirmation of these effects of AZM and CLM, a second proliferation test was performed, using T-cells labeled with a fluorescent tracer. Figure 1B presents the graphical results, demonstrating a similar suppressive effect for AZM at a concentration of 10 mg/L, as for 40 mg/L CLM (p = 0.475).
Suppression of CD4+ T-cells proliferation following activation.
(A) Median proliferation values (with quartile 1 and quartile 3) normalized to the respective antibiotic-free medium control from thymidine incorporation assays from CD4+ T-cells from ten apparently healthy volunteers; if not otherwise indicated, all statistical comparisons relate to the antibiotic-free medium control; n.s. not significant. (B) Proliferation of eFluor670 CPD labeled CD4+ T-cells 96 hours after stimulation in the presence of antibiotic-free medium or the indicated concentrations of AZM and CLM; numbers indicate division index; one representative experiment (n = 4) is depicted.
Suppression of T-cell cytokine secretion
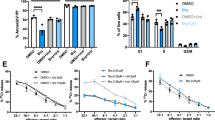
To further assess the influence of AZM and CLM on CD4+ T-cell function, the secretion of effector cytokines was measured in cell culture supernatants (Figure 2, Supplementary Table S1). Already at a concentration of 2.5 mg/L AZM, a significant reduction of IL-2, IL-10 (p = 0.002) and IFN-gamma (p < 0.001) was observed, whereas in presence of 2.5 mg/L CLM no significant change of cytokines was found. At an AZM concentration of 10 mg/L normalized mean levels of effector cytokines were reduced to 0.40 for IL-2 (p < 0.001), 0.34 for IL-10 (p < 0.001), 0.67 for IL-13 (p < 0.001), 0.53 for IL-17 (p < 0.001) and 0.50 for IFN-gamma (p < 0.001). In cells exposed to 10 mg/L CLM, solely IL-2 presented with a significant reduction to 0.78 mean expression (p = 0.006). At 40 mg/L AZM or CLM, secretion of all cytokines measured was further reduced (p-value range: <0.001 to 0.041). However, when comparing the suppressive potency of AZM and CLM at this concentration, a significant difference was found for IL-2 (p = 0.036), IL-10 (p = 0.003), IL-13 (p = 0.006), IL-17 (p = 0.017) and IFN-gamma (p = 0.003). Similar to proliferation, values obtained from the supernatant of T-cell exposed to 10 mg/L AZM were comparable to values obtained from those exposed to 40 mg/L CLM.
Cytokine production.
Mean secretion values (+SD) after 24 hours (IL-2) or 72 hours (IFN-gamma, IL-10, IL-13, IL-17) from triplicate cultures of CD4+ T-cells from one representative donor activated in the presence of the indicated concentrations of AZM (black bars) and clarithromycin CLM (white bars). Antibiotic-free medium and RAPA (100 nM) served as controls.
Dose dependent induction of apoptosis
To discriminate whether these immunosuppressive functions of AZM and CLM are mediated by the inhibition of activation or the induction of cell death, cell viability was assessed by apoptosis assays with CD4+ T-cells from eight healthy donors. The cell viability in antibiotic free medium treated cells 72 hours after activation ranged between 44 to 91%. No significant decrease in cell viability was observed at an AZM concentration of 2.5 mg/L (normalized median: 0.94, IQR: 0.87–1.03, p > 0.999, Figure 3A). At an AZM concentration of 10 mg/L, a slight decrease in viability of the cultures was measured (median: 0.85, IQR: 0.69–0.90), which was not found to be statistically significant (p = 0.139). Cell viability was strongly reduced to a median level of 0.45 (IQR: 0.26–0.46, p = 0.001), at an AZM concentration of 40 mg/L. In contrast, cellular viability in T-cells exposed to CLM was not significantly affected at all tested concentrations.
Dose dependent induction of apoptosis.
(A) Dot plots depicting Annexin V (x-axis) and propidium iodide (y-axis) stainings from CD4+ T-cells exposed to antibiotic-free medium or the indicated concentrations of AZM and CLM 72 hours after activation; data from one representative individual (n = 8) are depicted; numbers in the quadrants indicate respective percentage of viable cells (lower left), early apoptotic cells (lower right) and late apoptotic cells (upper right). (B) Relative caspase 3/7 activity of CD4+ T-cells exposed to antibiotic-free medium or the indicated concentrations of AZM and CLM 24 hours after activation; cumulative data (mean + SD) from four apparently healthy donors are depicted; n.s. not significant.
To further substantiate these observations, activation of the caspases 3 and 7 was assessed in responses to exposure to AZM and CLM. Similarly to the results obtained in the Annexin V/PI stainings, no significant increase in caspase 3/7 activity could be found at AZM concentrations of 2.5 mg/L (p = 0.932) and 10 mg/L (p = 0.863). At AZM 40 mg/L, caspase activity was approximately two-fold increased (p = 0.001). In contrast, exposure to CLM did not affect caspase activity in T-cells (Figure 3B).
Assessment of intracellular signaling pathways
In order to define the molecular mechanisms underlying the immunosuppressive function of AZM, we first assessed the effects of AZM on intracellular signaling pathways using Jurkat reporter cell lines. Exposure to 10 mg/L AZM did not affect NFAT (p = 0.999), NF-κB (p = 0.136) and AP1 promoter activity (p = 0.116). Comparable results were observed for exposure with 100 nM RAPA (p = 0.461, p = 0.275, p = 0.055, respectively). Specificity of the assays was confirmed by use of the calcineurin inhibitor Cyclosporin A (CsA) and the protein kinase C inhibitor GÖ6983 (Figure 4 and Supplementary Figure S2).
Assessment of NFAT, NF-kB and AP-1 signaling pathways.
Mean promoter activity values (+SD) from Jurkat T-cells activated in the presence of the indicated substances (AZM 10 mg/L; RAPA 100 nM; CsA 1 μM, GÖ6983 10 μM) normalized to the respective antibiotic-free medium control from four independent experiments.
Earlier reports have indicated that AZM inhibits phosphorylation of the MAP-kinase ERK in mononuclear cells24. In our experimental setting, no difference in the phosphorylation of ERK was observed between AZM treated T-cells and controls in four healthy donors in a time course of 60 minutes following activation (data not shown). In accordance, flow cytometric analyses of PMA-activated CD4+ T-cells showed that phosphorylation of the MAP-kinases ERK and p38 was not altered by AZM (Figure 5).
Assessment of MAP kinase signaling pathways.
Flow cytometry results for expression of phosphorylated ERK (left panels) or phosphorylated p38 (right panels) in PMA-activated T-cells treated with either antibiotic-free medium, 100 nM RAPA or 10 mg/L AZM (bold lines) and compared to unstimulated T-cells (fine lines). grey filled histograms: isotype control staining; values indicate mean fluorescence expression; one representative experiment (n = 3) is depicted.
Since these assays suggested that MAP-kinase and calcineurin-NFAT signaling are not inhibited by AZM, we subsequently investigated the effect of macrolides on the mTOR pathway. For that purpose the phosphorylation status of the S6 ribosomal protein (S6RP, a consensus mTOR target protein) was assessed in AZM, CLM, RAPA or antibiotic free medium exposed T-cells by flow cytometry (Figure 6A). T-cell activation resulted in a robust S6RP phosphorylation 24 hours after stimulation (33.9 to 45.1% positive cells, n = 4), which was nearly completely abrogated by RAPA. Exposure to AZM led to a significant stepwise reduction of the S6RP phosphorylation, starting with AZM 2.5 mg/L (mean: 0.92, p = 0.005). AZM 10 mg/L decreased phosphorylation to a normalized mean of 0.64 (p < 0.001), while AZM 40 mg/L led to a further reduction to 0.27 (p < 0.001). In contrast to AZM, CLM only showed a significant decrease at 40 mg/L (mean: 0.70, p < 0.001, Figure 6A).
Assessment of S6RP phosphorylation.
(A) Flow cytometry results for phosphorylation of S6RP at Ser240 in unstimulated T-cells and T-cells activated in the presence of antibiotic-free medium, 100 nM RAPA and the indicated concentrations of AZM and CLM; black line depicts specific staining; grey filled histograms depict staining with isotype-matched control antibody; numbers indicate percentage of positive cells; one representative experiment (n = 4) is depicted. (B) Immunoblotting results; whole cell-lysates were prepared from CD4+ T-cells after activation in the absence (antibiotic-free medium) or presence of azithromycin (AZM; 10 mg/L) at the indicated time points (minutes, min). Samples (both medium and AZM) were loaded on the same gel. After the transfer on a PVDF membrane, samples were first probed with an anti-phospho-S6RP antibody. Following band visualization, the membrane was further probed with a total anti-S6RP antibody. For presentation of data, the respective lanes taken from the same membrane were digitalized and cropped: phospho-S6RP (upper lanes) and total S6RP (lower lanes). Dagger and double-dagger sign indicate cropping from the same membrane lane. Numbers indicate phospho-S6RP/total S6RP ratio; one representative experiment (n = 4) is depicted; immunoblotting was performed under constant conditions for each blot, including resolving time, transfer time, antibody concentrations and exposure time. (C) Cumulative phospho-S6RP/total S6RP ratios determined by densitometry from four independently performed experiments; black: antibiotic-free medium, grey: AZM (10 mg/L).
Since the quality and magnitude of a T-cell response is to a great extent decided within the first hours following activation37, we also assessed S6RP phosphorylation in a time course of 60 minutes post stimulation. For these analyses, immunoblotting was used which has a higher sensitivity for protein phosphorylation than flow cytometry. As above, AZM treated CD4+ T-cells from four healthy donor presented with a significant reduction of the phosphorylation signal of S6RP compared to medium treated cells (p < 0.001). A first phosphorylation signal could be detected in medium treated cells after 20 minutes post activation and reached peak levels after 60 minutes. Similarly, a very weak phosphorylation signal of S6RP was observed in AZM treated cells after 20 minutes. However, overall signal strength did not reach the magnitude of medium treated cells at subsequent time points. This difference between AZM-treated and medium treated T-cells was especially pronounced 45 minutes (mean reduction: 44%, p = 0.032) and 60 minutes (mean reduction: 43%, p = 0.003) after activation (Figure 6B).
In order to further define the mechanism of action, we assessed the influence of AZM on the activity of recombinant mTOR in vitro using phosphorylation of a recombinant p70S6K-GST fusion protein as readout. Addition of 500 nM RAPA was used to validate the assay. In accordance with observations about the mechanism of action of RAPA, a strong suppression of mTOR activity (reduction 67.3%, p < 0.001) was found in the presence of recombinant FKBP12, while no influence on mTOR activity could be detected in the absence of FKBP12. In contrast, a dose-dependent inhibition of mTOR activity was measured in the presence of AZM, independently of the presence of recombinant FKBP12 (Figure 7). At a concentration of 1000 mg/L, AZM suppressed mTOR activity by 31.5% (p < 0.001) in the presence of FKBP12 and 27.0% (p < 0.001) in the absence of FKBP12 indicating that AZM acts as a direct mTOR kinase activity inhibitor. A major activating factor for mTOR is the phosphoinositide 3 kinase (PI3-K)34. Consequently, we also tested the effect of AZM on the activity of recombinant PI3-K using the generation of phosphatidyl-inositol 3 phosphate (PIP3) from phosphatidyl-inositol 2 phosphate (PIP2) as readout. Even at high concentrations (1000 mg/L) no inhibition (p = 0.6267) of PI3-K activity could be observed.
Assessment of mTOR and PI3-K activity in vitro.
(A) In vitro activity of recombinant mTOR; samples were treated with RAPA (500 nM) or indicated AZM concentration and normalized to positive control, grey: samples without FKBP12, black: samples with FKBP12; cumulative data from two independent experiments (mean + SD) analyzed in quadruplet are depicted; n.s.: not significant. (B) In vitro activity of recombinant PI3-K, samples were treated with the indicated AZM concentrations and normalized to the positive control; data are representative of mean + SD from quintuplet measurements; n.s.: not significant.
Discussion
The aim of this study was to investigate in vitro effects of AZM and CLM on human CD4+ T-cells. We observed that AZM acts as a potent suppressor of T-cell activation, whereas approximately four-fold higher levels of CLM are needed to achieve similar suppressive effects. Exposition to AZM and high levels of CLM decreased cell proliferation as well as secretion of effector cytokines. In case of AZM, this process was found to be dose-dependent. Cell viability assays confirmed that these effects were caused by specific immunosuppression and not by the induction of apoptosis. As a mechanism of action we identified that AZM inhibited mTOR kinase activity independently of FKBP12.
Several clinical studies on diseases with an auto-inflammatory or auto-immune background have described a therapeutic effect for AZM and CLM, which could not be explained by its anti-bacterial properties13,15,17. Interestingly, although T-cells are strongly involved in the regulation of virtually any immune response, the immunomodulatory effects of macrolides on T-cells have to date not been thoroughly characterized. In this respect, we have shown for the first time that AZM and CLM directly exert suppressive effects on the activation of purified CD4+ T-cells.
According to their cytokine secretion profile, CD4+ T-cell responses can be classified into different T-helper cell (Th) subsets. Several reports indicate that these diverse Th-subsets might have different sensitivities towards inhibition by immunosuppressive drugs38,39, although some drugs such as RAPA influence all Th-subsets40. Similarly, we found that AZM decreased secretion of all assessed cytokines. This indicates that AZM might have a general influence on CD4+ T-cells independently of their subset polarization. To further substantiate this observation, in-depth experiments with T-cells polarized towards distinct subsets are clearly needed. Although also implied as an immunomodulatory agent, only high concentrations of CLM had significant effects on the proliferation rate and for the most part on effector cytokine secretion of CD4+ T-cells. These findings are in line with a cytokine expression study in PMA/ionomycin activated T-cells using up to 125 mg/L CLM. There, CLM induced a slight reduction of intracellular IL-4 production, starting at levels of 20 mg/L CLM, whereas the IFN-gamma production was not altered41. The present findings suggest that the immunomodulatory potency of CLM in T-cells is less pronounced than the immunomodulatory potency of AZM, which is also supported by observations made in clinical studies. While the effects of AZM in the prevention of exacerbations in COPD or bronchiolitis obliterans syndrome after lung transplantation and in the treatment of asthma are consistent, several studies evaluating CLM in these indications could not demonstrate advantages regarding survival or clinical endpoints42,43,44.
The underlying question is whether the intrinsic inhibitory potencies of AZM and CLM were different or whether the observed differences of effects were based on altered intracellular concentrations in T-cells. In pharmacokinetic (PK) studies, tissue levels of AZM and CLM during standard therapy both range between 5 to 10 mg/L45. Moreover, macrolides show massive intracellular accumulation which depends on extracellular factors pH, temperature or an inflammatory environment as well as on the cell type8,9,10. So far, most studies have assessed intracellular concentration in preparations of mononuclear leukocytes and polymorphonuclear leukocytes. In contrast, there is a considerable lack of knowledge about pharmacological properties in purified lymphocytes fractions. Yet, it can be assumed that a several hundred-fold enrichment of macrolides can also be found in T-cells similar to other leukocytes. In this regard, investigations also into the in vivo enrichment of macrolides are highly warranted. Furthermore, apart from standard application, long-term, low dosage regimens for the therapy with macrolides exist, which may have distinct PK properties. Although low doses (0.6 mg/L) of AZM had no apparent direct effect on T-cell function in our short-term assays, further studies assessing long-term effects, are also needed. Regarding the mechanism of action, previous studies have shown that AZM is a strong inducer of apoptosis in lymphocytes30,31,32. However, in these studies, AZM concentrations exceed more than ten-fold the concentrations (up to 200 mg/L) usually found in tissues. In the present study, the effect of AZM on T-cell viability was investigated at concentrations (up to 10 mg/L) obtained in lymph nodes and lung tissue of individuals taking standard dosage46,47,48,49,50. At these levels T-cell viability following activation was only minimally reduced and caspase activity did not differ from T-cells in antibiotic free medium. When AZM levels were increased to 40 mg/L, a significant increase of apoptosis rate and caspase activity was observed, which is in line with the above mentioned reports. In contrast, CLM up to 40 mg/L had no influence on the viability and caspase activity of T-cells.
Several molecular signaling pathways have been described as immunomodulatory targets of AZM in innate immune cells, including the inhibition of ERK phosphorylation24. In the present study ERK phosphorylation was not altered by AZM in CD4+ T-cells within 60 minutes following stimulation. However, our observations need not necessarily contradict previous observations since substantial experimental differences exist. In a study by Hiwatashi et al., the modulation of ERK and JNK phosphorylation by AZM was analyzed after 48 hours24. Furthermore, peripheral blood mononuclear cells were assessed as opposed to highly purified CD4+ T-cells used by us. Consequently, their observations might reflect molecular events in monocytes or lymphocytes other than CD4+ T-cells.
Since our results suggest that NFAT and MAP kinase signaling are not affected by AZM, we analyzed the mTOR pathway, which is a major signaling pathway and crucial for nutrient, hormonal and co-stimulatory signal sensing in T-cells33. Using phosphorylation of a consensus mTOR target protein S6RP as readout, we showed that AZM and high concentrations of CLM interfered with mTOR signaling. In these signaling assays, the immunosuppressive capacity of 10 mg/L AZM and 40 mg/L CLM, reached approximately 50 percent of the suppression mediated by RAPA. Interestingly, this effect was sufficient to achieve nearly similar results on cell proliferation measured in thymidine incorporation assays (Supplementary Figure 1).
In order to further define the molecular effect of AZM on mTOR signaling we performed an in vitro kinase assay using phosphorylation of the recombinant p70S6K by a recombinant catalytic fragment of mTOR as readout. In these experiments we could show that AZM down-regulates mTOR activity at a concentration of 1000 mg/L by approximately 30%, which roughly correlates with the suppression of functional effects. Given that pharmacokinetic studies have indicated that AZM gets enriched in leukocytes more than 100-fold above serum levels6,7, it is highly likely that the relevant mTOR inhibitory intracellular concentrations are reached in CD4+ T-cells treated with 10 mg/L AZM in the culture medium. In contrast to RAPA, we found this inhibition of mTOR kinase activity to be independent of the presence of FKBP12. Thus, although AZM shares structural features with RAPA, AZM directly acts on the mTOR protein without the need for co-factors. In this respect it will be interesting to further analyze the interaction between AZM and the mTOR protein using bio-structural methods. Activity of mTOR is governed by a multitude of upstream signaling molecules. Among these, the PI3-kinase acts as a major upstream mTOR activator. Previous studies have implied that treatment with AZM leads to activation of PI3-K51,52. However, using an in vitro kinase assay, we could not observe a direct effect of AZM on PI3-K activity. Thus, the observations found in these studies might be due to indirect signaling effects.
The clinical implications of our observations could be far-reaching. Aside from its use as an immunosuppressive agent following solid organ transplantation, mTOR inhibitors such as RAPA are nowadays used in the therapy of proteinuric glomerulopathies, polycystic kidney disease, refractory uveitis and different types of cancer and may have beneficial effects in atherosclerosis as well as neurological diseases including multiple sclerosis and autoimmune encephalomyelitis36,53,54,55,56. Due to its similar molecular target, it is tempting to speculate that AZM might be administered for similar clinical indications with the additional benefit of offering a well-defined antibiotic spectrum. Furthermore, in contrast to other macrolides, AZM does not inhibit the human CYP3A4 oxidases1, thus avoiding an interference with other drugs.
On the other hand, the immunosuppressive potential of AZM might become detrimental in situations of impaired immune function as found in conditions of sepsis or HIV infection. According to current guidelines, AZM and CLM are the first line prophylaxis against atypical mycobacterium avium complex (MAC) in HIV positive patients with a CD4 count less than 50 cells/μl23 For that purpose, no significant difference between the efficacies of AZM and CLM was found in a recent meta-analysis57. However, it might be hypothesized, that administration of AZM might prolong the lymphopenic phase and might therefore represent an additional mortality risk factor58. Thus, further studies on the duration of lymphopenia in HIV patients in relation to the administration of macrolides are warranted.
Septic patients frequently present with an overwhelming reaction of the immune response and therefore the administration of macrolides has shown beneficial results in septic patients, especially when the sepsis is secondary to pneumonia59,60. A suppressed immune status marked by lymphopenia61, associated with a higher mortality rate62, is also frequently observed in the course of a severe sepsis. In particular, CD4+ T-cells are affected from an increased apoptosis rate, leading to an elevated risk of viral co-infection63,64,65. In the light of the immunosuppressive function of AZM and CLM, it might be necessary to re-evaluate respective treatment regimens. Therefore, it might be preferable to switch to other antibiotics which do not suppress the effector functions of CD4+ T-cells. In this context, further in vitro and in vivo studies with other commonly used antibiotics (e.g. beta-lactam antibiotics, fluorochinolones, macrolides) are necessary.
In conclusion, a remarkable difference in the immunosuppressive potency between AZM and CLM on highly purified CD4+ T-cells was shown for the first time. These effects of AZM seem to be translated by specific inhibition of mTOR kinase activity in an FKBP12 independent fashion. These present findings warrant further studies assessing the clinical impact of the immunosuppressive effects observed in various patient groups.
Methods
Ethical considerations, cell isolation and culture
The study was approved by the local ethics committee of the Medical University of Vienna (EC number 1724/2012) and conducted according to the Declaration of Helsinki (1969, including current revisions) of the World Medical Association. After obtaining informed consent of study participants, peripheral blood mononuclear cells (PBMC) were isolated from ten healthy volunteers (8 male, 2 female).
CD4+ T-cells were isolated from PBMC using the Human CD4+ T-cell Isolation Kit II (Miltenyi Biotech, Bergisch Gladbach, Germany) according to the manufacturers' instructions. Purity was assessed by flow cytometric analyses using monoclonal antibodies against human CD3 (clone OKT3, eFluor 450 conjugated; eBioscience, San Diego, CA) and CD4 (clone OKT4, FITC conjugated; eBioscience) and routinely found to be above 95%. All functional assays were performed in IMDM (GE Healthcare, Piscataway, NJ) supplemented with 10% fetal calf serum (GE Healthcare), 2 mM L-glutamine and 0.1 mM 2-mercaptoethanole (Sigma Aldrich, St. Louis, MO).
Proliferation assays
Proliferation assays were performed as previously described66. In short, CD4+ T-cells (1 × 105) were stimulated in 96-well flat bottom plates with anti-CD3/anti-CD28 coated microbeads (0.5 × 105; Invitrogen, Carlsbad, CA) for 72 hours, pulsed with [methyl-3H] thymidine (1 μCi per well, Perkin Elmer, Boston, MA) for additional 18 hours before being harvested and processed on a Packard scintillation counter (Packard, Meriden, CT). The indicated concentrations of AZM (Zitromax®, Pfizer, New York, NY), CLM (Klacid®, Abbott Laboratories, Abbott Park, IL) or the respective purified substances (Sigma Aldrich) were added 60 minutes prior to stimulation. T-cells stimulated in antibiotic-free medium served as controls. AZM and CLM were analyzed at four concentration levels to simulate peak concentrations in various body compartments46,47,48,49,50. In some experiments, CD4+ T-cells were labeled with the eFluor670 cell proliferation dye (1.5 μM; eBioscience) and activated as above. For these experiments, cellular proliferation was determined on a FACS Calibur flow cytometer (BD Biosciences, Franklin Lakes, NJ). After stringent gating on viable cells according to their forward-scatter/side-scatter properties, cells were analyzed using the Cell Quest software (BD Biosciences, Version 3.3). For comparison of proliferation data from CPD-labeled effector T-cells, a division index (DI) was calculated according to the following formula67:

Determination of cytokine secretion
CD4+ T-cells (1 × 106) were stimulated in 24-well flat bottom plates with anti-CD3/anti-CD28 coated microbeads (0.5 × 106) in the presence of the indicated concentrations of AZM, CLM or antibiotic-free medium. Due to different expression kinetics of cytokines following activation, supernatants of CD4+ T-cells were harvested after 24 and 72 hours and concentrations of the indicated cytokines were determined by multiplex analysis (Luminex 100IS; Biomedica, Vienna, Austria). Briefly, IL-2 secretion, representing T-helper (Th) cell activation of all subsets, was measured after 24 hours. Th-1 activity was measured by interferon gamma (IFN-gamma) secretion after 72 hours. As an indicator for Th-2 activity, the secretion of cytokines of IL-4 and IL-13 and for Th17 activity IL-17 was assessed after 72 hours. Additionally, IL-10 levels, which are attributed to Th-2 and regulatory T-cells, were measured.
Determination of cell death and caspase activity
CD4+ T-cells (1 × 106) were stimulated in 24-well flat bottom plates with anti-CD3/anti-CD28 coated microbeads (0.5 × 106) in the presence of the indicated concentrations of AZM, CLM or in antibiotic-free medium. After 72 hours, cells were harvested, washed once in staining buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4; Sigma Aldrich). Annexin V FITC (5 μl; Bender MedSystems, Vienna, Austria) was added and cells were incubated at room temperature for 10 minutes. After washing cells once in staining buffer, propidium iodide (PI, Sigma Aldrich) was added (final concentration 100 ng/mL) and cells were analyzed on a FACS Calibur flow cytometer using Cell Quest software. Annexin V negative/PI negative cells were quantified and identified as viable cells.
For measurement of caspase 3/7 activity the luciferase-based Caspase-Glo 3/7 Assay (Promega, Madison, WI) was used according to the manufacturers' instructions. CD4+ T-cells (1 × 106) were stimulated in 24-well flat bottom plates with anti-CD3/anti-CD28 coated microbeads (0.5 × 106) in the presence of the indicated concentrations of AZM, CLM or in antibiotic-free medium. After 24 hours, cells were harvested and 5 × 104 cells were seeded in 50 μL in triplicates into a 96-well luciferase measurement plate. After addition of 50 μL of the reaction substrate, cells were incubated for 2 hours at room temperature in the dark. Afterwards, luciferase was determined as readout for caspase activity. Values were normalized to the mean caspase activity obtained in T-cells activated in antibiotic-free medium.
Screening of NFAT, NF-κB and AP-1 signaling in Jurkat reporter cell lines
In the presence or absence of AZM (10 mg/L) and well-defined inhibitors including RAPA (100 nM), CsA (1 μM) and GÖ6983 (10 μM), 106 Jurkat T-cell expressing an enhanced green fluorescence protein (eGFP) reporter gene under control of either an NFAT, NF-κB or AP-1 promoter element were stimulated with 0.2 × 106 T cell stimulator cells expressing CD80 (TCS-CD80). T cell stimulator cells were used instead of microbead stimulation (as described above), since Jurkat T-cells have a higher activation threshold than peripheral blood CD4+ T-cells. This discrepancy is based on a lower expression of cell surface receptors on the Jurkat T-cells line. The TCS-CD80 cells are based on Bw5147 cells (murine thymoma) transfected with a membrane-bound OKT3- (anti-CD3-) single-chain fragment and human CD80 and have been described in detail in Leitner et al.68. After 24 hours the percentage of eGFP positive cells was measured by flow cytometry and used to determine promoter activity.
Flow cytometric analyses of intracellular signaling pathways
5 × 105 CD4+ T-cells were stimulated in 24-well flat bottom plates with either phorbol-12-myristate-13-acetate (PMA; 10−7 M; Sigma Aldrich) for 30 minutes or anti-CD3/anti-CD28 coated microbeads (2.5 × 105) for 24 hours in the presence of 10 mg/L AZM, 100 nM RAPA or in antibiotic-free medium. Subsequently, cells were harvested and incubated for ten minutes in Fixation Buffer I (BD Phosflow, BD Biosciences) at 37°C. After washing in PBS + 0.5% BSA + 0.05% NaN3 cells were resuspended in pre-chilled (−20°C) Permeabilization Buffer III (BD Phosflow, BD Biosciences) and kept on ice for 30 minutes followed by washing the cells twice in PBS + 0.5% BSA + 0.05% NaN3. Then, cells were incubated with one of the following antibodies: 20 μL of a p-ERK (T202/Y204; Pacific Blue conjugated; clone 20A), p-p38 (T180/Y182; PE conjugated; clone 36/p38) or p-S6RP (S240; Alexa Fluor 647 conjugated; clone N4-41; all BD Phosflow, BD Biosciences) specific monoclonal antibodies or isotype matched control antibodies for one hour. After washing in PBS + 0.5% BSA + 0.05% NaN3 cells were cells were analyzed on a FACS Canto flow cytometer using FlowJo software (TreeStar, Ashland, OR).
Biochemical analyses
CD4+ T-cells (4 × 106) were stimulated in 24-well flat bottom plates with anti-CD3/anti-CD28 coated microbeads (2 × 106) in the presence of 10 mg/L AZM or in antibiotic-free medium. At the indicated time points cells were harvested, collected by centrifugation (700 × g, 5 min) and lysed in RIPA buffer supplemented with phosphatase- and protease inhibitors (Sigma Aldrich). Cellular debris was removed by centrifugation at 25.000 × g and 4°C for 15 minutes. Samples were normalized according to their protein content and were resolved by SDS-PAGE on 4–12% gradient gels under reducing conditions (Life Technologies, Paisley, UK) followed by transfer onto PVDF membranes (GE Healthcare). Samples were then subjected to immunoblotting using the following antibodies: anti-phospho-S6 ribosomal protein (S240/244; rabbit; clone D68F8), anti-S6 ribosomal protein (rabbit; clone 5G10), anti-phospho-MAPK (ERK1/2) (T202/Y204; rabbit; clone D13.14.4E) and anti-ERK (rabbit polyclonal, all Cell Signaling Technology, Danvers, MA). After incubation with a secondary anti-rabbit horse radish peroxidase-conjugated antibody, binding was visualized using the SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL). Signal strength was quantified by densitometry using the ImageJ software (National Institute of Health, Bethesda, MD; Version 1.47). Mean intensities of immunoblot bands were measured and background intensity was subtracted. For each time point and condition a ratio of phosphorylated S6RP to total S6RP was calculated from these corrected values.
In vitro kinase assays
For measurement of the influence of macrolides on mTOR activity, the K-LISA mTOR Activity Kit (Merck Millipore, Darmstadt, Germany) was used according to the manufacturers' instructions. In short, the indicated concentrations of AZM or 500 nM RAPA were pre-incubated on ice in the presence or absence of recombinant FKBP12 (3 μg per sample) for 20 minutes. Plain buffer and FKBP12 without antibiotics served as negative and positive control respectively. A recombinant mTOR fragment (amino acids 1360–2549, 50 ng per sample) containing the kinase domain was added for another 20 minutes. Afterwards, samples were transferred to microtiter plates pre-coated with a p70S6K-GST fusion protein (500 ng per well) and incubated at 30°C for 30 minutes. Subsequently, mTOR-mediated phosphorylation of p70S6K was determined in an ELISA using a monoclonal antibody specific for p70S6K phosphorylated at Thr389.
Effects of AZM on the PI3-K were examined using the PI3-kinase activity ELISA (Echelon, Salt Lake City, UT). In short, the indicated concentrations of AZM were added to 50 ng recombinant PI3-K (p110α/p85α; Echelon) and conversion of PIP2 substrate to PIP3 was performed for 2 hours at 37°C. Afterwards, concentrations of PIP3 were determined using a competitive ELISA system.
Statistical analyses
Statistical analysis was performed using MedCalc (MedCalc Software bvba, Ostend, Belgium, Version 12.7.7.0) and GraphPad Prism (GraphPad Software Inc., La Jolla, CA, Version 6.02). Proliferation rates, cytokine and cell viability levels were normalized to controls (cells activated in antibiotic-free medium) and are given in percentages. Depending on the presence of absence of a normal distribution, parameters are given as mean with standard deviation (SD) or as median with interquartile range (IQR). For group comparisons, a paired t-test or a Wilcoxon signed rank test and an analysis of variance (ANOVA) or a Kruskal-Wallis test was applied. For post-hoc comparison, the non-parametric Dunn's or the Holm-Sidak's multiple comparisons test, both with adjusted p-values, were used. Statistical significance was defined as a p-value (two-sided) less than 0.05. In figures, significant values are denoted as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001. Where appropriated, the Bonferroni-Holm method was applied to correct for type I errors related to multiple testing.
References
Zarogoulidis, P. et al. Macrolides: from in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur J Clin Pharmacol 68, 479–503 (2012).
Balloy, V. et al. Azithromycin analogue CSY0073 attenuates lung inflammation induced by LPS challenge. Br J Pharmacol. 171, 1783–1794 (2014).
Poehlsgaard, J. & Douthwaite, S. The bacterial ribosome as a target for antibiotics. Nat. Rev. Microbiol. 3, 870–881 (2005).
Zuckerman, J. M., Qamar, F. & Bono, B. R. Macrolides, ketolides and glycylcyclines: azithromycin, clarithromycin, telithromycin, tigecycline. Infect Dis Clin North Am. 23, 997–1026 (2009).
Hand, W. L. & Hand, D. L. Characteristics and mechanisms of azithromycin accumulation and efflux in human polymorphonuclear leukocytes. Int. J. Antimicrob. Agents 18, 419–425 (2001).
Steel, H. C., Theron, A. J., Cockeran, R., Anderson, R. & Feldman, C. Pathogen- and host-directed anti-inflammatory activities of macrolide antibiotics. Mediators Inflamm. 2012, 584262 (2012).
Wildfeuer, A., Laufen, H. & Zimmermann, T. Uptake of azithromycin by various cells and its intracellular activity under in vivo conditions. Antimicrob Agents Chemother. 40, 75–79 (1996).
Fietta, A., Merlini, C. & Gialdroni Grassi, G. Requirements for intracellular accumulation and release of clarithromycin and azithromycin by human phagocytes. J Chemother 9, 23–31 (1997).
Ballow, C. H., Amsden, G. W., Highet, V. S. & Forrest, A. Pharmacokinetics of Oral Azithromycin in Serum, Urine, Polymorphonuclear Leucocytes and Inflammatory vs Non-Inflammatory Skin Blisters in Healthy Volunteers. Clin Drug Investig. 15, 159–167 (1998).
Amsden, G. W. & Gray, C. L. Serum and WBC pharmacokinetics of 1500 mg of azithromycin when given either as a single dose or over a 3 day period in healthy volunteers. J Antimicrob Chemother. 47, 61–66 (2001).
Albert, R. K. et al. Azithromycin for Prevention of Exacerbations of COPD. N Engl J Med. 365, 689–698 (2011).
Simoens, S., Laekeman, G. & Decramer, M. Preventing COPD exacerbations with macrolides: A review and budget impact analysis. Respir. Med. 107, 637–648 (2013).
Wolter, J. et al. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 57, 212–216 (2002).
Schultz, M. J. Macrolide activities beyond their antimicrobial effects: macrolides in diffuse panbronchiolitis and cystic fibrosis. J Antimicrob Chemother. 54, 21–28 (2004).
Wong, C. et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet 380, 660–667 (2012).
Yang, M. et al. Macrolides for diffuse panbronchiolitis. The Cochrane database of systematic reviews 2, Cd007716 (2013).
Gottlieb, J. et al. Long-term azithromycin for bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 85, 36–41 (2008).
Maimon, N., Lipton, J. H., Chan, C. K. N. & Marras, T. K. Macrolides in the treatment of bronchiolitis obliterans in allograft recipients. Bone Marrow Transplant. 44, 69–73 (2009).
Brusselle, G. G. et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 68, 322–329 (2013).
Simpson, J. L., Powell, H., Boyle, M. J., Scott, R. J. & Gibson, P. G. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med. 177, 148–155 (2008).
Gotfried, M. H. Macrolides for the treatment of chronic sinusitis, asthma and COPD. Chest 125, 52S–61S (2004).
Opal, S. M. New perspectives on immunomodulatory therapy for bacteraemia and sepsis. Int J Antimicrob Agents. 36, S70–S73 (2010).
National Guideline, C. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. Recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health and the HIV Medicine Association of the Infectious Diseases Society of America, 2013 URL: http://www.guideline.gov/content.aspx?id=45359 (accessed: 09.09.2014)
Hiwatashi, Y. et al. Azithromycin suppresses proliferation, interleukin production and mitogen-activated protein kinases in human peripheral-blood mononuclear cells stimulated with bacterial superantigen. J Pharm Pharmacol 63, 1320–1326 (2011).
Ikegaya, S., Inai, K., Iwasaki, H., Naiki, H. & Ueda, T. Azithromycin reduces tumor necrosis factor-alpha production in lipopolysaccharide-stimulated THP-1 monocytic cells by modification of stress response and p38 MAPK pathway. J Chemother 21, 396–402 (2009).
Khan, A. A., Slifer, T. R., Araujo, F. G. & Remington, J. S. Effect of clarithromycin and azithromycin on production of cytokines by human monocytes. Int J Antimicrob Agents. 11, 121–132 (1999).
Vrancic, M. et al. Azithromycin distinctively modulates classical activation of human monocytes in vitro. Br J Pharmacol. 165, 1348–1360 (2012).
Bosnar, M. et al. Azithromycin inhibits macrophage interleukin-1 beta production through inhibition of activator protein-1 in lipopolysaccharide-induced murine pulmonary neutrophilia. Int Immunopharmacol. 11, 424–434 (2011).
Vanaudenaerde, B. M. et al. Macrolides inhibit IL17-induced IL8 and 8-isoprostane release from human airway smooth muscle cells. Am J Transplant 7, 76–82 (2007).
Kadota, J.-I. et al. Antibiotic-induced apoptosis in human activated peripheral lymphocytes. Int J Antimicrob Agents 25, 216–220 (2005).
Ishimatsu, Y. et al. Macrolide antibiotics induce apoptosis of human peripheral lymphocytes in vitro. Int J Antimicrob Agents 24, 247–253 (2004).
Mizunoe, S. et al. Clarithromycin and azithromycin induce apoptosis of activated lymphocytes via down-regulation of Bcl-xL. Int Immunopharmacol 4, 1201–1207 (2004).
Mondino, A. & Mueller, D. L. mTOR at the crossroads of T cell proliferation and tolerance. Semin Immunol. 19, 162–172 (2007).
Bi, Y. J., Liu, G. W. & Yang, R. F. mTOR Regulates T-Cell Differentiation and Activation in Immunity and Autoimmunity. Crit. Rev. Eukaryot. Gene Expr. 21, 313–322 (2011).
Morath, C. et al. Sirolimus in renal transplantation. Nephrol Dial Transplant 22 Suppl 8viii61–viii65 (2007).
Buhaescu, I., Izzedine, H. & Covic, A. Sirolimus - Challenging current perspectives. Ther. Drug Monit. 28, 577–584 (2006).
Lee, K. H. et al. T cell receptor signaling precedes immunological synapse formation. Science 295, 1539–1542 (2002).
Egli, A., Kumar, D., Broscheit, C., O'Shea, D. & Humar, A. Comparison of the effect of standard and novel immunosuppressive drugs on CMV-specific T-cell cytokine profiling. Transplantation. 95, 448–455 (2013).
Kim, W. U. et al. Divergent effect of cyclosporine on Th1/Th2 type cytokines in patients with severe, refractory rheumatoid arthritis. J Rheumatol. 27, 324–331 (2000).
Kawamoto, K., Pahuja, A., Hering, B. J. & Bansal-Pakala, P. Transforming growth factor beta 1 (TGF-beta1) and rapamycin synergize to effectively suppress human T cell responses via upregulation of FoxP3+ Tregs. Transpl Immunol. 23, 28–33 (2010).
Williams, A. C., Galley, H. F., Watt, A. M. & Webster, N. R. Differential effects of three antibiotics on T helper cell cytokine expression. J Antimicrob Chemother. 56, 502–506 (2005).
Dhillon, G. S. et al. Clarithromycin for prevention of bronchiolitis obliterans syndrome in lung allograft recipients. Clin. Transplant. 26, 105–110 (2012).
Sutherland, E. R. et al. A trial of clarithromycin for the treatment of suboptimally controlled asthma. J Allergy Clin Immunol. 126, 747–753 (2010).
Banerjee, D., Khair, O. A. & Honeybourne, D. The effect of oral clarithromycin on health status and sputum bacteriology in stable COPD. Respir. Med. 99, 208–215 (2005).
Zeitlitzger, M., Waper, C. C. & Heinisch, B. Ketolides - The Modern Relatives of Macrolides The Pharmacokinetic Perspective. Clin Pharmacokinet 48, 23–38 (2009).
Cazzola, M. et al. Evaluation of lung tissue and hilar lymph node concentrations of azithromycin. Int J Clin Pharmacol Ther. 32, 88–91 (1994).
Rodvold, K. A., Gotfried, M. H., Danziger, L. H. & Servi, R. J. Intrapulmonary steady-state concentrations of clarithromycin and azithromycin in healthy adult volunteers. Antimicrob Agents Chemother. 41, 1399–1402 (1997).
Amsden, G. W. & Gray, C. L. Serum and WBC pharmacokinetics of 1500 mg of azithromycin when given either as a single dose or over a 3 day period in healthy volunteers. J Antimicrob Chemother. 47, 61–66 (2001).
Liu, P. et al. Comparative pharmacokinetics of azithromycin in serum and white blood cells of healthy subjects receiving a single-dose extended-release regimen versus a 3-day immediate-release regimen. Antimicrob Agents Chemother. 51, 103–109 (2007).
Matzneller, P. et al. Blood, Tissue and Intracellular Concentrations of Azithromycin during and after End of Therapy. Antimicrob Agents Chemother. 57, 1736–1742 (2013).
Stamatiou, R. et al. Azithromycin has an antiproliferative and autophagic effect on airway smooth muscle cells. Eur Respir J. 34, 721–730 (2009).
Stamatiou, R., Boukas, K., Paraskeva, E., Molyvdas, P. A. & Hatziefthimiou, A. Azithromycin reduces the viability of human bronchial smooth muscle cells. J Antibiot 63, 71–75 (2010).
Chen, W. Q. et al. Oral rapamycin attenuates inflammation and enhances stability of atherosclerotic plaques in rabbits independent of serum lipid levels. Br J Pharmacol. 156, 941–951 (2009).
Zhao, L. et al. Low-dose oral sirolimus reduces atherogenesis, vascular inflammation and modulates plaque composition in mice lacking the LDL receptor. Br J Pharmacol. 156, 774–785 (2009).
Dello Russo, C., Lisi, L., Feinstein, D. L. & Navarra, P. mTOR kinase, a key player in the regulation of glial functions: relevance for the therapy of multiple sclerosis. Glia. 61, 301–311 (2013).
Liu, Y. M., Shao, Y. Q. & He, Q. Sirolimus for treatment of autosomal-dominant polycystic kidney disease: a meta-analysis of randomized controlled trials. Transplant Proc. 46, 66–74 (2014).
Uthman, M. M. B., Uthman, O. A. & Yahaya, I. Interventions for the prevention of mycobacterium avium complex in adults and children with HIV. Cochrane Database Syst Rev. 4, Cd007191 (2013).
Seage, G. R. et al. The relationship of preventable opportunistic infections, HIV-1 RNA and CD4 cell counts to chronic mortality. J. Acquir. Immune Defic. Syndr. 30, 421–428 (2002).
Pereira, J. M., Paiva, J. A. & Rello, J. Severe sepsis in community-acquired pneumonia - Early recognition and treatment. Eur. J. Intern. Med. 23, 412–419 (2012).
Giamarellos-Bourboulis, E. J. Immunomodulatory therapies for sepsis: unexpected effects with macrolides. Int J Antimicrob Agents. 32, S39–S43 (2008).
Inoue, S. et al. Reduction of immunocompetent T cells followed by prolonged lymphopenia in severe sepsis in the elderly. Crit Care Med. 41, 810–819 (2013).
Boomer, J. S. et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306, 2594–2605 (2011).
Roth, G. et al. Susceptibility to programmed cell death in T-lymphocytes from septic patients: a mechanism for lymphopenia and Th2 predominance. Biochem Biophys Res Commun. 308, 840–846 (2003).
Osawa, R. & Singh, N. Cytomegalovirus infection in critically ill patients: a systematic review. Critical Care. 13, R68 (2009).
Walton, A. H. et al. Reactivation of multiple viruses in patients with sepsis. PloS One. 9, e98819 (2014).
Schmetterer, K. G. et al. Bet v 1-specific T-cell receptor/forkhead box protein 3 transgenic T cells suppress Bet v 1-specific T-cell effector function in an activation-dependent manner. J Allergy Clin Immunol. 127, 238–245, 245.e231–233 (2011).
Asquith, B. et al. Quantifying lymphocyte kinetics in vivo using carboxyfluorescein diacetate succinimidyl ester (CFSE). Proc Biol Sci. 273, 1165–1171 (2006).
Leitner, J. et al. T cell stimulator cells, an efficient and versatile cellular system to assess the role of costimulatory ligands in the activation of human T cells. J Immunol Methods. 362, 131–141 (2010).
Acknowledgements
We want to acknowledge Ms. Sabine Belik for invaluable technical assistance. The study was conducted in cooperation with the MedUni Wien Biobank facility. The project was supported by grants of the Austrian Society for Laboratory Medicine and Clinical Chemistry (ÖGLMKC); the Medical Scientific Fund of the Mayor of the City of Vienna [Grant 13040BGM, 14041BGM]; and the Austrian Science Fund [Grant SFB F4609]. None of the founders had influence on any phase of this project.
Author information
Authors and Affiliations
Contributions
F.R., W.P., P.S., H.B., W.F.P. and K.G.S. participated in research design, F.R., G.H., J.J.K. and K.G.S. performed functional, biochemical and flow cytometric analyses, H.H., G.H., J.J.K., S.J. and W.F.P. contributed reagents and analytical tools and participated in data analysis and interpretation, all authors wrote and critically revised the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Ratzinger, F., Haslacher, H., Poeppl, W. et al. Azithromycin suppresses CD4+ T-cell activation by direct modulation of mTOR activity. Sci Rep 4, 7438 (2014). https://doi.org/10.1038/srep07438
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07438
This article is cited by
-
Clarithromycin impairs tissue-resident memory and Th17 responses to macrolide-resistant Streptococcus pneumoniae infections
Journal of Molecular Medicine (2021)
-
Effects of pre-transplant azithromycin administration on kidney graft function: study protocol for a double-blind randomized clinical trial
Trials (2018)
-
Piperine enhances carbohydrate/fat metabolism in skeletal muscle during acute exercise in mice
Nutrition & Metabolism (2017)
-
First-line antibiotic therapy in Helicobacter pylori-negative low-grade gastric mucosa-associated lymphoid tissue lymphoma
Scientific Reports (2017)
-
Chloroquine inhibits human CD4+ T-cell activation by AP-1 signaling modulation
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.