Article Text
Statistics from Altmetric.com
Mounting evidence indicates that chronic obstructive pulmonary disease (COPD) is an independent risk factor for cardiovascular (CV) mortality. Data from several large trials such as the Lung Health Study, Towards a Revolution in COPD Health, Understanding Potential Long-term Impacts on Function with Tiotropium, European Respiratory Society Study on Chronic Obstructive Pulmonary Disease and Inhaled Steroids in Obstructive Lung Disease (ISOLDE) have shown that fatal CV events contribute to a major portion (between 22% and 39%) of the deaths in this population.1 Patients with COPD have greater mortality, rehospitalisation rates and poorer health status after a myocardial infarction (MI). Recent insights into the mechanisms that underlie this excess risk support a strong biological basis for heightened susceptibility to CV events, with mechanisms such as oxidative stress, endothelial dysfunction, arterial stiffness, increased inflammation and thrombotic predisposition being common themes.2 While a biological basis for inherent susceptibility of patients with COPD for CV events is indeed highly likely, inequities in their medical care resulting in substantial ‘gaps’ in treatment have also been suggested to contribute to the disproportionate mortality.3 ,4 While the reasons are likely multifactorial, at least one plausible explanation relates to differences in symptom presentations of acute coronary syndrome (ACS) in COPD, where patients may present with dyspnoea, rather than chest pain, obfuscating the diagnosis of MI and in turn delaying institution of appropriate management (eg, reperfusion therapies).3
In their Heart paper, Rothnie et al provide fascinating insights into the associations between COPD and excess mortality following a first MI using data from the Myocardial Ischaemia National Audit Project (MINAP), a national register for all MI and ACS admissions in the UK.5 MINAP records information on patient comorbidity, care processes and management for both ST-segment elevation MI (STEMI) and non-STEMI (NSTEMI), and thus provides an opportunity to evaluate the impact of these factors on follow-up mortality. Indeed, prior contributions from MINAP have included the recognition of reduced use of secondary prevention strategies in patients with COPD and the fact that the use of β-blockers in this patient population started either before or after MI is associated with improve survival.6 Delays in the diagnosis of STEMI, institution of reperfusion therapy after STEMI, use of angiography after NSTEMI and use of secondary prevention medications were investigated. As observed in other similar cohorts, patients with COPD were more likely to be older, to be current or past smokers and more likely to have comorbidities such as peripheral arterial disease, cerebrovascular disease and heart failure.7
The investigators found that both in-hospital and 6-month mortality were higher among patients with COPD with a STEMI (OR 1.24, (95% CIs 1.10 to 1.41) and 1.45 (95% CI 1.32 to 1.54) respectively) after adjustments for demographics, comorbidities and medication usage on arrival. Patients with COPD were 25%–50% more likely to have an initial diagnosis other than definite STEMI or ACS, suggesting that atypical presentations may be a contributor to the delay in recognition. However, reperfusion time with primary percutaneous coronary intervention (PCI) was delayed only among patients with COPD with a delayed diagnosis (153 min (IQR, 74–706) vs 109 min (IQR, 50–260 min) for non-COPD patients). The excess risk of in-hospital mortality was roughly cut in half and no longer statistically significant after further adjustments for diagnostic delay, time to reperfusion and use of primary PCI (OR 1.11, 95% CI 0.94 to 1.31). This translated into a decrease in the estimated attributable risk of in-hospital death due to COPD from 19.4% (95% CI 9.1 to 29.1%) to 11.5% (95% CI −0.01 to 22.4%). On the other hand, the heightened mortality risk at 6 months following STEMI in patients with COPD was not decreased after adjusting for in-hospital management factors, but rather was markedly attenuated after accounting for the usage of secondary prevention medications upon discharge (from 43% down to 25% excess risk).
Patients with COPD presenting with NSTEMI also had higher adjusted risks for in-hospital and 6-month mortality. They were more likely to have an initial diagnosis other than ACS and less likely to receive angiography as well as secondary prevention medications upon discharge. In-hospital mortality risk was cut by over 50% after adjusting for delays and use of angiography; however, the residual excess risk remained statistically significant compared with non-COPD patients. Similarly, the higher 6-month mortality risk was substantially reduced, yet persisted after adjustment of in-hospital processes and secondary prevention medications (1.26, 95% CI 1.17 to 1.35).
The findings from this analysis of MINAP are reminiscent of results from the ACTION Registry, where chronic lung disease including COPD in 155 786 patients was independently associated with an increased risk of in-hospital mortality in the setting of NSTEMI but not STEMI. Similarly, data from the SWEDEHEART registry revealed that patients with COPD and with MI were less likely to receive diagnostic angiography, percutaneous intervention and secondary prevention medications. The risk associated with COPD and MI was markedly attenuated in both studies after adjustment in MI care processes and secondary medication use. However, this current analysis of MINAP sheds important additional light on the impact of care processes in a contemporary data set and provides novel insights regarding the effects of several in-hospital factors versus medical care upon discharge on mortality following STEMI and NSTEMI.
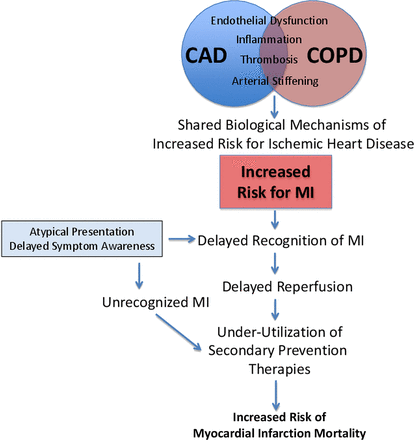
A few limitations beyond those noted by the investigators include the lack of information on the timing of adjunctive pharmacotherapies, as delays in diagnosis may have also resulted in delays in institution of such therapies. Information on whether patients presented with an initial diagnosis of COPD exacerbation, a condition known to be associated with increased risk for MI, is also not available. The impact of other factors that might plausibly differ between groups such as the severity and anatomical location of MI, extent of underlying coronary atherosclerosis, arrhythmia burden, shock/hypotension and concomitant hypoxaemia could not be assessed. Recidivism rates among former smokers and exposure to second-hand smoke might be higher at 6 months among patients with COPD. Follow-up medication adherence rates may also differ. Finally, the reasons for lower prescription rates for medications (eg, statins, angiotensin-converting enzyme inhibitors) other than β-blockers in patients with COPD are not readily apparent and may reflect some confounding health issues (eg, frailty, fall risk, prior side effects). A propensity-matched analysis may have allowed the covariates to be better balanced and may provide better distinction of risk. Regardless, these results are important and provide much needed detail on pathways to identify and reduce risk in patients with COPD. It is worth highlighting that this analysis was performed only in patients with COPD who actually had an MI confirmed. In the real world setting, the atypical presentation of COPD may result in the missed diagnosis of MI altogether, thereby translating into a much higher risk for mortality (figure 1).
Inter-relationships between COPD and heightened cardiovascular risk. CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction.
The results from this analysis of MINAP reiterate the significance of COPD as an important determinant of acute and 6-month prognosis following MI. The findings also provide compelling support that this ‘mortality gap’ in patients with COPD presenting with MI may be ‘mended’ to a substantive degree through scrupulous attention to hospital care process and the fastidious institution of appropriate secondary prevention therapies. On the other hand, the outcome ‘gap’ was lessened but not eliminated. This strongly supports that other factors including biological susceptibility also underlie the relationship between COPD and residual risk for mortality. Given the rising global burden of COPD and the persistent associations with excess CV events, there are compelling reasons to consider targeting COPD-specific disease pathways causally related to a potentiated CV disease process. From this perspective, the ongoing Study to Understand Mortality and Morbidity in COPD (the SUMMIT study) is a particularly timely trial.8 SUMMIT is assessing the effect of combination inhaled COPD therapy (ie, fluticasone furoate/vilanterol vs placebo) on all-cause mortality (primary outcome) as well as fatal and non-fatal CV events (secondary outcomes) in patients with moderate COPD with either a prior history of CV disease or with multiple risks for it. Therapies that address biological mechanisms common to COPD and ischaemic CV diseases (eg, inflammation) as well as other treatments that target COPD-specific pathways (eg, lung function) may prove to be important in the global effort to lessen the heightened CV morbidity and mortality attributable to COPD.
Footnotes
Contributors SR and Brook made substantial contributions to the conception or design of the work, analysis and interpretation of data and to drafting the article or revising it critically for important intellectual content.
Competing interests None declared.
Provenance and peer review Commissioned; internally peer reviewed.