Abstract
Objective
Neurally adjusted ventilatory assist (NAVA) is a new mode wherein the assistance is provided in proportion to diaphragm electrical activity (EAdi). We assessed the physiologic response to varying levels of NAVA and pressure support ventilation (PSV).
Setting
ICU of a University Hospital.
Patients
Fourteen intubated and mechanically ventilated patients.
Design and protocol
Cross-over, prospective, randomized controlled trial. PSV was set to obtain a V t/kg of 6–8 ml/kg with an active inspiration. NAVA was matched with a dedicated software. The assistance was decreased and increased by 50% with both modes. The six assist levels were randomly applied.
Measurements
Arterial blood gases (ABGs), tidal volume (V t/kg), peak EAdi, airway pressure (Paw), neural and flow-based timing. Asynchrony was calculated using the asynchrony index (AI).
Results
There was no difference in ABGs regardless of mode and assist level. The differences in breathing pattern, ventilator assistance, and respiratory drive and timing between PSV and NAVA were overall small at the two lower assist levels. At the highest assist level, however, we found greater V t/kg (9.1 ± 2.2 vs. 7.1 ± 2 ml/kg, P < 0.001), and lower breathing frequency (12 ± 6 vs. 18 ± 8.2, P < 0.001) and peak EAdi (8.6 ± 10.5 vs. 12.3 ± 9.0, P < 0.002) in PSV than in NAVA; we found mismatch between neural and flow-based timing in PSV, but not in NAVA. AI exceeded 10% in five (36%) and no (0%) patients with PSV and NAVA, respectively (P < 0.05).
Conclusions
Compared to PSV, NAVA averted the risk of over-assistance, avoided patient–ventilator asynchrony, and improved patient–ventilator interaction.
Similar content being viewed by others
Introduction
Modes of partial ventilatory assistance are preferred to reduce side-effects and complications associated with controlled mechanical ventilation. With these modes, ventilator cycling is ideally under control of the patient’s own respiratory drive and rhythm, which also influences the ventilatory output to an extent that varies with the different modes [1]. Coordination between spontaneous breathing and mechanical assistance, however, is not guaranteed and a poor interaction between patient and machine may represent a major problem in the ventilatory management of patients with acute respiratory failure [2, 3].
One of the most widely used forms of assisted ventilation is pressure support ventilation (PSV), wherein a preset level of pressure assists every spontaneous inspiration. Although PSV has been proven to be valuable in several clinical conditions [4, 5], dyssynchrony between spontaneous inspiration and mechanical cycling has been frequently described in terms of prolonged mechanical insufflation [6–9], ineffective efforts [2, 3, 9], premature interruption of mechanical insufflation [3] and double triggering [3]. Asynchrony may be secondary to multiple factors, including machine characteristics and performance, patient’s breathing pattern, inspiratory muscle strength, mechanical properties of the respiratory system, and factors affecting neural respiratory drive, such as level of sedation, acidosis and fever. The amount of assistance is also a determinant of asynchrony; in particular, excessive levels of inspiratory support may worsen patient–ventilator interaction [7, 10, 11].
Neurally adjusted ventilatory assist (NAVA) is a new form of partial support wherein the machine applies positive pressure throughout inspiration in proportion to the electrical activity of the diaphragm (EAdi), as assessed by trans-esophageal electromyography [12]. The amount of assistance for a given EAdi depends on a user-controlled gain factor [12, 13]. With intact phrenic nerves, EAdi is the earliest and best signal available to estimate the neural respiratory drive [12]. Because ventilator functioning and cycling are under control of the patient’s respiratory drive and rhythm, NAVA has the potential to enhance patient–ventilator interaction ensuring synchrony and minimizing the risk of over-assistance. However, because sedatives and other factors potentially depressing the central nervous system, NAVA might, in principle, decrease alveolar ventilation, especially for low levels of assistance.
The aim of this study is to compare, in ventilator-dependent ICU patients, the physiologic response to varying levels of ventilator assistance in PSV and NAVA.
Materials and methods
The study was performed in the Intensive Care Unit (ICU) of the Azienda Ospedaliera Maggiore della Carità (Novara, Italy), according to the principles outlined in the Declaration of Helsinki. The institutional ethics committee approved the study. Patient consent was obtained according to the Italian regulations [14].
Subjects
All intubated patients receiving partial ventilatory support were considered eligible. Exclusion criteria were: (1) age <18 years, (2) gastro-esophageal surgery in the previous 12 months, (3) gastro-esophageal bleeding in the previous 30 days, (4) history of esophageal varices, (5) facial trauma and/or surgery, (6) hemodynamic instability despite adequate filling [i.e. need for continuous infusion of epinephrine or vasopressin, or dopamine >5 γ/(kg min) or norepinehrine >0.1 γ/(kg min) to maintain systolic arterial blood pressure >90 mmHg], (7) core temperature >38°C, (8) coagulation disorders (INR ratio >1.5 and PTT >44 s), (9) inability to maintain a tidal volume (V t) ≤ 8 ml/kg with a minimum inspiratory support of 8 cmH2O, and (10) inclusion in other research protocol.
General principles of NAVA
The study was performed using a prototype Servo-I ventilator (Maquet Critical Care, Sölna, Sweden) capable of delivering pressure control ventilation, PSV, and NAVA. As previously described [15], EAdi was obtained through a nasogastric tube with a multiple array of electrodes placed at its distal end (EAdi catheter, Maquet Critical Care, Sölna, Sweden). Correct positioning of the EAdi catheter was assured by means of a specific function of the ventilator (“EAdi catheter positioning”).
The EAdi signal is processed according to the American Thoracic Society (ATS) recommendations [16] and filtered by algorithms designed to provide the highest possible signal-to-noise ratio. To avoid interference secondary to variations in lung volume and chest wall configuration [16], changes in diaphragm position along the array are also considered [13, 15]. EAdi is quantified every 16 ms using the root-mean-square [12, 15]. Portions of signal with residual disturbances are removed and replaced by the values of the previous segment [17].
The amount of pressure instantaneously applied by the ventilator to the airway opening throughout inspiration is determined by the processed EAdi, expressed in μV, multiplied by a user-controlled gain factor (“NAVA level”), whose unit is cmH2O/μV. The shape of the airway pressure (Paw) therefore resembles the EAdi profile, while the amount of assistance depends on the magnitude of both EAdi and “NAVA level”. The ventilator can be cycled-on by two different algorithms, based on either EAdi, or Paw or flow, according to a hierarchy that follows the principle that “first-serves-first”. During NAVA, the ventilator is cycled-off when the EAdi falls at 70% of its peak inspiratory value.
Study protocol
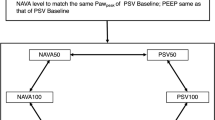
Upon patient enrollment, the ventilator currently in use and the conventional nasogastric tube were replaced with the aforementioned prototype ventilator and “EAdi catheter”, respectively. Correct positioning of the catheter was assessed as mentioned above. We titrated the inspiratory pressure support to obtain a V t of 6–8 ml/kg with an active inspiration, as assessed by a positive rise of 2–4 cmH2O in Paw at the end of an end-inspiratory occlusion [18]. The flow-trigger sensitivity was adjusted at the lowest possible level without auto-triggering, while the expiratory trigger threshold was 30% of the peak inspiratory flow. We defined this level of assistance PSV100. Then, we determined the corresponding NAVA level (NAVA100) using a dedicated function of the prototype ventilator (“NAVA Preview”), which provides during PSV an estimate of the NAVA level achieving an equivalent peak inspiratory Paw (Paw,peak) [19]. PSV100 and NAVA100 were then increased (PSV150 and NAVA150) and decreased (PSV50 and NAVA50) by 50%. PEEP and inspired oxygen fraction (FiO2) were maintained constant throughout the study period at the values in use prior to patient enrollment.
Each patient underwent three 20-min trials, as indicated for physiological assessment [20], in each mode. The six trials were performed in random order, following predefined computer-generated random sequences. The last 5 min of each trial were recorded and stored on a dedicated personal computer for further data analysis. At the end of each trial, arterial blood was sampled for gas analysis from a catheter which had been previously placed in the radial artery for the purpose of monitoring. All patients were sedated with propofol, either alone or in association with opiates (remifentanil or fentanyl) to obtain a Ramsay sedation scale value of 3 [21]. The infusion of sedatives was maintained constant throughout the study period. The endotracheal tube was suctioned before the beginning of each trial.
Criteria for protocol discontinuation were: (1) hemodynamic instability (mean arterial blood pressure <60 mmHg), (2) agitation, (3) oxygen saturation <90%, or (4) peak Paw > 40 cmH2O.
Data acquisition and analysis
Airflow, Paw and EAdi were acquired from the ventilator through a RS232 interface at a sampling rate of 100 Hz, recorded by means of dedicated software (Nava Tracker V. 2.0, Maquet Critical Care, Sölna, Sweden), and analyzed using a customized software based on Microsoft Excel®. Paw,peak and peak of EAdi swing (EAdi,peak) were measured. The integral of Paw over time (PTPaw) was calculated. From the flow signal we obtained ventilator rate of cycling (RRflow); flow-based inspiratory (T i,flow) and expiratory time (T e,flow); total breath duration (T tot,flow); neural inspiratory duty cycle (T e/T tot,flow); and V t by digital integration. From the EAdi signal we calculated patient’s own (neural) respiratory rate (RRneu), inspiratory (Ti,neu) (i.e. time between onset of EAdi swing and EAdi,peak) and expiratory time Te,neu (i.e. time between EAdi,peak and onset of the following EAdi swing), and neural inspiratory duty cycle (T i/T tot,neu). Inspiratory trigger delay was calculated as the difference in time between the onsets of EAdi and Paw inspiratory swings. The coefficient of variation (CV) for V t, RRflow, EAdi,peak, and Paw,peak was also calculated (standard deviation to mean ratio multiplied by 100) as previously described [22, 23]. To estimate the extent of dyssynchrony we used the asynchrony index (AI) [2, 3]. AI expresses in percentage the number of asynchrony events (ineffective efforts and double triggering) divided by the total respiratory rate (i.e. the sum of ventilator cycles and ineffective efforts) [2, 3]. An AI > 10% is considered a high rate of asynchrony [2, 24].
Data were compared using the analysis of variance (ANOVA) for repeated measures. The Bonferroni post hoc test was used to compare equivalent levels of assistance (PSV50 vs. NAVA50, PSV100 vs. NAVA100 and PSV150 vs. NAVA150). Changes at different levels of assistance within each mode (PSV50 vs. PSV100 vs. PSV150 and NAVA50 vs. NAVA100 vs. NAVA150) were assessed by means of the post hoc test for linear trend analysis. The slopes of the linear correlations between EAdi,peak and V t, and EAdi,peak and Paw,peak at each level of assistance were also calculated and compared using ANOVA. AI was compared between the two modes using Fisher’s exact test.
Results
We enrolled 16 consecutive patients; two of them, however, did not complete the study and could not be included in the data analysis. Both excluded patients interrupted the study protocol because of peak Paw > 40 cmH2O during the NAVA150 trials. Anthropometric and clinical characteristics of the 14 patients who concluded the study protocol are shown in Table 1.
Mean levels of assistance and PEEP are provided in Table 2. As also shown in Table 2, arterial pH, arterial oxygen tension to fraction of inspired oxygen ratio (PaO2/FiO2), and arterial carbon dioxide tension (PaCO2) were not significantly different between PSV and NAVA at all levels of assistance. Also, pH, PaO2/FiO2 and PaCO2 showed no linear trend among levels of assistance within either mode.
Figure 1 depicts in one representative subject the effects of varying the assistance provided with PSV and NAVA on flow, Paw, V t, and EAdi. As presented in Table 2, V t/kg was not significantly different between either PSV100 and NAVA100 and PSV50 and NAVA50; compared to NAVA150, however, V t/kg was significantly higher during PSV150 (P < 0.05), which was the only trial wherein V t/kg exceeded 8 ml/kg. Both RRneu and RRflow were significantly lower with PSV150 than with NAVA150 (P < 0.05). Both RRneu and RRflow showed a significant reduction when increasing the assistance with PSV (P < 0.001), but not with NAVA.
Examples of tracings from one representative patient breathing with PSV and NAVA. From top to bottom, flow, Paw, V t, and EAdi are shown. Low (PSV50 and NAVA50), middle (PSV100 and NAVA100), and high (PSV150 and NAVA150) assist levels are displayed from left to right. Differences between PSV and NAVA were relatively small at the two lower assist levels. On the contrary, as opposed to NAVA150, PSV150 resulted in smaller EAdi, higher tidal volume, and occurrence of ineffective efforts. Paw, airway pressure; V t, tidal volume; EAdi, diaphragm electrical activity; PSV, pressure support ventilation; NAVA, neurally adjusted ventilatory assist
Figure 2a illustrates T i,neu (upper panel) and T i,flow (lower panel) changes produced by varying the assistance with the two modes. Irrespective of the mode of ventilation, T i,neu did not change when varying the assistance. There was no difference in T i,neu between PSV and NAVA at all levels of assistance. T i,flow showed a similar behavior with NAVA, while it progressively rose increasing PSV (P < 0.05), so that it was significantly longer during PSV150, compared to NAVA150 (P < 0.05). Figure 2b depicts T e,neu (upper panel) and T e,flow (lower panel) changes produced by the two modes. T e,neu significantly augmented increasing PSV, but not NAVA, and was significantly longer at PSV150, compared to NAVA150. T e,flow also did not change varying NAVA level, while it progressively increased at higher PSV (P < 0.05) so that it was significantly longer during PSV150, compared to NAVA150 (P < 0.05). As shown in Table 2, T i/T tot,neu did not vary when modifying NAVA level, while it significantly decreased when rising PSV (P < 0.01). T i/T tot,neu was smaller with PSV150 than with NAVA150 (P < 0.01); T i/T tot,flow was significantly reduced at increasing levels of assistance with PSV (P < 0.01), but not with NAVA. Nevertheless, there was no significant difference in T i/T tot,flow between PSV and NAVA at any assist level.
a Group mean data (mean ± SD) of neural (T i,neu) and flow-based (T i,flow) inspiratory time with PSV (filled triangle) and NAVA (hollow circle) at the three support levels are shown in the upper and lower panel, respectively. With NAVA, both T i,neu and T i,flow did not change when varying the level of support. With PSV, T i,neu also remained unchanged when varying the level of support, while T i,flow rose increasing support and was significantly higher at PSV150, compared to NAVA150. b Group mean data (mean ± SD) of neural (T e,neu) and flow-based (T e,flow) inspiratory time with PSV (filled triangle) and NAVA (hollow circle) at the three support levels are shown in the upper and lower panel, respectively. With NAVA, both T e,neu and T e,flow did not change when varying the level of support. With PSV, T e,neu and T e,flow both rose with increasing support and were significantly higher at PSV150, compared to NAVA150. Pressure support ventilation (PSV); neurally adjusted ventilatory assist (NAVA). * P < 0.05, NAVA versus PSV at equivalent level of assistance. § P < 0.05, linear trend for NAVA or PSV
As displayed in Table 2, with both modes Paw,peak and PTPaw significantly rose when increasing support (P < 0.001). Paw,peak was not significantly different between the two modes at corresponding levels of assistance, while PTPaw was higher with PSV150 than with NAVA150 (P < 0.001). As also shown in Table 2, with both modes EAdi,peak decreased after increasing assistance (P < 0.001). EAdi,peak was significantly lower at PSV150 than at NAVA150 (P < 0.05).
Figure 3 depicts individual data of EAdi,peak versus Paw,peak (panel a) and EAdi,peak versus V t (panel b) relationships in one representative subject, for PSV (upper panels) and NAVA (lower panels). In PSV, regardless of EAdi, the support delivered by the ventilator (Paw,peak) did not vary. Conversely, in NAVA Paw,peak changed in proportion to EAdi (Fig. 3a). In PSV, V t slightly rose when increasing EAdi only at the lowest level of support; in NAVA, V t augmented as EAdi increased at all levels of assistance (Fig. 3b). Group mean data for the slopes of EAdi,peak versus Paw,peak and EAdi,peak versus V t are provided in Table 2. For both relationships, the slopes significantly rose at increasing levels of assistance only with NAVA (P < 0.01). The EAdi,peak versus Paw,peak slope was significantly higher at NAVA100 (P < 0.01) and NAVA150 (P < 0.001), as opposed to PSV100 and PSV150, respectively. The EAdi,peak versus V t slope was significantly higher at NAVA150 than at PSV150 (P < 0.01). Figure 4 depicts the relationships between group mean data (±SD) of V t and EAdi for PSV and NAVA, at all levels of assistance. Reducing ventilator assistance by 50% caused an increase in EAdi and a slight reduction in V t with both modes. When increasing the assistance by 50%, a further decrease in EAdi and a remarkable rise in V t occurred with PSV, while both V t and EAdi showed little changes with NAVA.
Relationships between Paw and EAdi (a), and tidal volume (V t) and EAdi (b) are depicted for the three assist levels with PSV (upper panels) and NAVA (lower panels), in one representative patient. a Paw did not change when varying the support level in PSV. Oppositely, in NAVA, Paw,peak varied in proportion to EAdi. b During PSV, V t slightly rose when increasing EAdi only at the lowest level of support. During NAVA, V t augmented as EAdi increased at all levels of assistance. Paw, airway pressure; V t, tidal volume; EAdi, diaphragm electrical activity; PSV, pressure support ventilation; NAVA, neurally adjusted ventilatory assist
Relationships between group mean data (mean ± SD) of V t and EAdi are shown for PSV (triangles) and NAVA (circles), at all levels of assistance. Group mean data (mean ± SD) for PSV100 and NAVA100 are indicated by grey symbols. V t was similar with the two modes, while EAdi was slightly, but not significantly, lower with PSV than with NAVA. When reducing ventilator assistance by 50% (black symbols), both modes showed an increase in EAdi and a slight reduction in V t. When augmenting the assistance by 50% (white symbols), PSV resulted in a further decrease in EAdi and a remarkable rise in V t, while NAVA produced little changes with respect to both variables. Please, refer to Table 2 for the statistical significance of the variables plotted in the graph. Paw, airway pressure; V t, tidal volume; EAdi, diaphragm electrical activity; PSV, pressure support ventilation; NAVA, neurally adjusted ventilatory assist
The CV of V t, RRflow, Paw,peak, and EAdi,peak did not change when varying the NAVA level (Table 2). During PSV there was a significant increase in the CV of RRflow (P < 0.01) and EAdi,peak (P < 0.05) at increasing levels of assistance. Compared to PSV100 and PSV150, the CV of V t was higher with NAVA100 and NAVA150 (P < 0.001). Both RRflow and EAdi,peak were higher with PSV150, as opposed to NAVA150 (P < 0.05).
Double triggering never occurred. Ineffective efforts were observed during PSV, but never during NAVA. AI exceeded 10% in five (36%) patients with PSV and in no patients with NAVA (P < 0.05). One patient showed ineffective efforts at all levels of PSV, two patients at the intermediate and high PSV level, and two patients only at PSV150.
Discussion
We found that irrespective of the assistance, arterial blood gases were not significantly different between PSV and NAVA. The differences in breathing pattern, ventilator assistance, and respiratory drive and timing between PSV and NAVA were small at the two lower assist levels, but significant at the highest assist. In the range of settings studied, compared to PSV, NAVA limited the risk of over-assistance, avoided patient–ventilator asynchrony, and improved overall patient–ventilator interaction.
When introducing a new mode of ventilation it is necessary to compare it with the established treatment, which in our case was PSV. We set the reference PSV (PSV100) in order to achieve a V t between 6 and 8 ml/kg. During PSV, however, the patient might produce a small, brief effort, just sufficient to trigger the ventilator and then relax, therefore being passively ventilated for the large majority of the inspiratory phase and not retaining control of his/her breathing pattern [25]. To overcome this drawback we used the non-invasive technique described by Foti et al. [18]. Moreover, when comparing modes of ventilation, equivalent levels of assistance should be used making the choice of the matching variable crucial. This choice, however, is not simple and is anyway susceptible to criticisms [26]. We opted for using the specific function (NAVA Preview) available on the ventilator to estimate the NAVA level necessary to deliver the assistance equivalent to a given preset PSV. PSV100 and NAVA100 did not show significant differences in Paw,peak (Table 2) and mean inspiratory Paw (18.2 ± 4.3 and 16.1 ± 5.1, for PSV100 and NAVA100, respectively, P = 0.23); moreover, V t/kg, breathing frequency, EAdi and PTPaw also showed no significant difference between PSV100 and NAVA100.
We included patients with different underlying diseases who had been receiving invasive mechanical ventilation for a variable time. We are aware that evaluating a more selected population of patients might result in more homogeneous results. In a previous study aimed to evaluate whether and to what extent the underlying disease affected diaphragmatic effort and patient–ventilator synchrony, Nava et al. [5] found that the application of different levels of PSV resulted in comparable diaphragmatic efforts in patients with acute respiratory failure due to different pathologies. Also, patient–ventilator asynchrony occurred with all underlying diseases, although it was more pronounced in patients with chronic obstructive pulmonary disease [5]. To date, no study reporting the use of NAVA in ICU patients, rather than in animals or healthy subjects, has been published yet.
Patients receiving mechanical ventilation require sedation and analgesia for anxiety and pain experienced during the time they are intubated. Accordingly, all patients included in our study received a continuous infusion of propofol, either alone or in association with remifentanil, to maintain a Ramsay score of 3 [21]. By depressing the neural drive, which controls the ventilator during NAVA, sedatives might more markedly affect NAVA than PSV. We found that PSV and NAVA had similar effects on gas exchange, irrespective of the assist level, suggesting that NAVA was not associated with an increased risk of hypoventilation secondary to sedative infusion. A total of 3 patients received propofol alone and 11 received both propofol and remifentanil. No patient received fentanyl. Propofol infusion rate ranged between 1 and 4 mg/(kg h); remifentanil infusion rate ranged between 0.05 and 0.2 μg/(kg min). Mean infusion rates were 2.2 ± 1.3 mg/(kg h) and 0.12 ± 0.05 μg/(kg min) for propofol and remifentanil, respectively. Noteworthy, in no patient propofol infusion exceeded 4 mg/(kg h) and only in three patients remifentanil infusion exceeded 0.125 μcg/(kg min). Indeed, the risk of central drive depression at these dosages is very low. We cannot exclude, however, that deeper levels of sedation might determine different results. Moreover, our protocol does not address whether or not varying the rate of sedative infusion would affect the two modes.
It has been repeatedly reported that increasing the level of PSV augments V t, decreases breathing frequency, neural drive, and inspiratory effort, and may worsen patient–ventilator synchrony [7, 10, 11]. We found that with both modes increasing the assist level produced significant increments in PTPaw and V t, and reduction in EAdi. While, at the two lower assist levels PSV and NAVA showed no significant differences in either the amount of assistance provided (i.e. PTPaw), breathing pattern, or neural drive (i.e. EAdi,Peak), PSV150 significantly increased V t and reduced breathing frequency, compared to NAVA150. Also, although Peak Paw values were not different between PSV150 and NAVA150, with the former, as opposed to the latter, the ventilator assistance was greater and the neural drive smaller. These results indicate that, overall, compared to PSV, NAVA has the potential to limit the risk of over-assistance.
Both neural- and flow-based respiratory timing was unaffected by the NAVA level. T i,neu was also unaffected by the support level in PSV. Increasing PSV, however, significantly lengthened T i,flow. With PSV, a prolonged mechanical insufflation exceeding T i,neu frequently occurs, especially at higher levels of support [1, 8, 27]. This is recognized to be the underlying mechanism of patient–ventilator asynchrony [28]. When the onset of the neural expiration is impeded, the response of the respiratory centers is to prolong the expiratory time [6, 29]. Accordingly, we found that increasing PSV lengthened both T e,neu and T e,flow. As a result, we observed significant differences in T i,flow, T e,neu, T e,flow, and T i/T tot,neu between PSV150 and NAVA150. Because T i/T tot,flow, was unchanged between the two modes regardless of the level of assistance, NAVA did not increase, when compared to PSV, the risk of dynamic hyperinflation.
Patient–ventilator asynchrony is a maladaptation of the ventilator to patient’s neural respiratory timing [28]. As expected, with NAVA we could not observe any asynchrony, regardless of the level of assistance. On the contrary, more than one-third of the PSV trials were characterized by ineffective efforts, as evidenced by an AI > 10%, with a rate that increased at the highest level of assistance. As recent work showed that patients with an AI exceeding 10% are subject to longer duration of mechanical ventilation and increased recourse to tracheostomy [3], these findings may be clinically relevant. Clearly, our results are valid in the range of setting studied and one could argue that further increasing the NAVA level might produce an additional decrease in EAdi and cause ineffective efforts to occur. Also, because of the mixed patient population, we chose not to vary the expiratory trigger threshold and leave the ventilator default value; we cannot exclude a lower rate of AI had occurred varying this PSV setting on an individual basis. Moreover, we do not know whether the use of an EAdi-based trigger during PSV would contain the occurrence of ineffective efforts and how the overall physiologic response to PSV would be consequently affected.
In a comparison between PSV and proportional assist ventilation (PAV), Wrigge et al. [23] found that V t variability was higher during PAV and rose when increasing the assist level. The authors considered these results as a consequence of the improved ability to vary V t by modulating the inspiratory effort. In our study, when compared to PSV, NAVA showed a higher V t variability regardless of the assist level. EAdi variability in NAVA was lower than in PSV, a difference that achieved statistical significance at the highest assist level. Furthermore, in contrast to the aforementioned study, when increasing the assistance the variability in breathing frequency progressively increased with PSV and decreased with NAVA. This was statistically significant when comparing PSV150 to NAVA150. Overall, as opposed to PSV, increasing NAVA augmented V t variability and reduced RRflow variability, mimicking the behavior of healthy individuals [22].
Conclusions
In summary, our study indicates that the response to changes in assist level is different between PSV and NAVA, especially at the highest assist. These differences, nonetheless, have little impact on gas exchange. In the range of settings studied, compared to PSV, NAVA shows the potential to limit the risk of over-assistance, prevent patient–ventilator asynchrony, and improve overall patient–ventilator interaction. As a whole, however, the differences in respiratory drive and timing, amount of ventilator assistance, and breathing pattern were remarkable only at the highest assist level. Finally, it is worth noting that this is a preliminary physiologic study and that further physiologic studies and clinical trials are necessary to clarify whether or not NAVA really represents an advantage compared to other forms of partial support in general and PSV in particular.
References
Younes M (1991) Proportional assist ventilation and pressure support ventilation: similarities and differences. In: Marini J, Roussos C (eds) Ventilatory failure. Springer, Berlin, pp 361–380
Chao DC, Scheinhorn DJ, Stearn-Hassenpflug M (1997) Patient–ventilator trigger asynchrony in prolonged mechanical ventilation. Chest 112:1592–1599
Thille AW, Rodriguez P, Cabello B, Lellouche F, Brochard L (2006) Patient–ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med 32:1515–1522
Cereda M, Foti G, Marcora B, Gili M, Giacomini M, Sparacino ME, Pesenti A (2000) Pressure support ventilation in patients with acute lung injury. Crit Care Med 28:1269–1275
Nava S, Bruschi C, Fracchia C, Braschi A, Rubini F (1997) Patient-ventilator interaction and inspiratory effort during pressure support ventilation in patients with different pathologies. Eur Respir J 10:177–183
Younes M, Kun J, Webster K, Roberts D (2002) Response of ventilator-dependent patients to delayed opening of exhalation valve. Am J Respir Crit Care Med 166:21–30
Leung P, Jubran A, Tobin MJ (1997) Comparison of assisted ventilator modes on triggering, patient effort, and dyspnea. Am J Respir Crit Care Med 155:1940–1948
Younes M, Puddy A, Roberts D, Light RB, Quesada A, Taylor K, Oppenheimer L, Cramp H (1992) Proportional assist ventilation. Results of an initial clinical trial. Am Rev Respir Dis 145:121–129
Giannouli E, Webster KIM, Roberts DAN, Younes M (1999) Response of ventilator-dependent patients to different levels of pressure support and proportional assist. Am J Respir Crit Care Med 159:1716–1725
Nava S, Bruschi C, Rubini F, Palo A, Iotti G, Braschi A (1995) Respiratory response and inspiratory effort during pressure support ventilation in COPD patients. Intensive Care Med 21:871–879
Appendini L, Purro A, Patessio A, Zanaboni S, Carone M, Spada E, Donner CF, Rossi A (1996) Partitioning of inspiratory muscle workload and pressure assistance in ventilator-dependent COPD patients. Am J Respir Crit Care Med 154:1301–1309
Sinderby C, Navalesi P, Beck J, Skrobik Y, Comtois N, Friberg S, Gottfried SB, Lindstrom L (1999) Neural control of mechanical ventilation in respiratory failure. Nat Med 5:1433–1436
Navalesi P, Costa R (2003) New modes of mechanical ventilation: proportional assist ventilation, neurally adjusted ventilatory assist, and fractal ventilation. Curr Opin Crit Care 9:51–58
Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G (2006) Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 354:1775–1786
Sinderby C, Beck J, Spahija J, de Marchie M, Lacroix J, Navalesi P, Slutsky AS (2007) Inspiratory muscle unloading by neurally adjusted ventilatory assist during maximal inspiratory efforts in healthy subjects. Chest 131:711–717
Aldrich T, Sinderby C, McKenzie D (2002) Electrophysiologic techniques for the assessment of respiratory muscle function. In: ATS/ERS (eds) Statement on respiratory muscle testing. Am J Respir Crit Care Med 166:518–624
Allo JC, Beck JC, Brander L, Brunet F, Slutsky AS, Sinderby CA (2006) Influence of neurally adjusted ventilatory assist and positive end-expiratory pressure on breathing pattern in rabbits with acute lung injury. Crit Care Med 34:2997–3004
Foti G, Cereda M, Banfi G, Pelosi P, Fumagalli R, Pesenti A (1997) End-inspiratory airway occlusion: a method to assess the pressure developed by inspiratory muscles in patients with acute lung injury undergoing pressure support. Am J Respir Crit Care Med 156:1210–1216
(2006) Ventilate with NAVA. In: AB MCC (eds) SERVO-I V32 NAVA ventilation, user’s manual
Fauroux B, Isabey D, Desmarais G, Brochard L, Harf A, Lofaso F (1998) Nonchemical influence of inspiratory pressure support on inspiratory activity in humans. J Appl Physiol 85:2169–2175
Ramsay MA, Savege TM, Simpson BR, Goodwin R (1974) Controlled sedation with alphaxalone–alphadolone. Br Med J 2:656–659
Tobin MJ, Mador MJ, Guenther SM, Lodato RF, Sackner MA (1988) Variability of resting respiratory drive and timing in healthy subjects. J Appl Physiol 65:309–317
Wrigge H, Golisch W, Zinserling J, Sydow M, Almeling G, Burchardi H (1999) Proportional assist versus pressure support ventilation: effects on breathing pattern and respiratory work of patients with chronic obstructive pulmonary disease. Intensive Care Med 25:790–798
Vitacca M, Bianchi L, Zanotti E, Vianello A, Barbano L, Porta R, Clini E (2004) Assessment of physiologic variables and subjective comfort under different levels of pressure support ventilation. Chest 126:851–859
Berger KI, Sorkin IB, Norman RG, Rapoport DM, Goldring RM (1996) Mechanism of relief of tachypnea during pressure support ventilation. Chest 109:1320–1327
Ambrosino N (1999) Apples and oranges. Comparing different modalities of mechanical ventilation. Intensive Care Med 25:775–777
Yamada Y, Du HL (2000) Analysis of the mechanisms of expiratory asynchrony in pressure support ventilation: a mathematical approach. J Appl Physiol 88:2143–2150
Sassoon CS, Foster GT (2001) Patient–ventilator asynchrony. Curr Opin Crit Care 7:28–33
Kondili E, Prinianakis G, Anastasaki M, Georgopoulos D (2001) Acute effects of ventilator settings on respiratory motor output in patients with acute lung injury. Intensive Care Med 27:1147–1157
Acknowledgments
We thank Maquet Critical Care that provided us with all the equipment used for the study. We are indebted to Jeffrey Franc-Law who carefully revised the manuscript. Last but not least, we wish to acknowledge all physicians and nurses of our Intensive Care Unit for their helpful and continuous support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at: doi:10.1007/s00134-008-1215-4.
Rights and permissions
About this article
Cite this article
Colombo, D., Cammarota, G., Bergamaschi, V. et al. Physiologic response to varying levels of pressure support and neurally adjusted ventilatory assist in patients with acute respiratory failure. Intensive Care Med 34, 2010–2018 (2008). https://doi.org/10.1007/s00134-008-1208-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1208-3