Abstract
Purpose
Advances in supportive care and ventilator management for acute respiratory distress syndrome (ARDS) have resulted in declines in short-term mortality, but risks of death after survival to hospital discharge have not been well described. Our objective was to quantify the difference between short-term and long-term mortality in ARDS and to identify risk factors for death and causes of death at 1 year among hospital survivors.
Methods
This multi-intensive care unit, prospective cohort included patients with ARDS enrolled between January 2006 and February 2010. We determined the clinical characteristics associated with in-hospital and 1-year mortality among hospital survivors and utilized death certificate data to identify causes of death.
Results
Of 646 patients hospitalized with ARDS, mortality at 1 year was substantially higher (41 %, 95 % CI 37–45 %) than in-hospital mortality (24 %, 95 % CI 21–27 %), P < 0.0001. Among 493 patients who survived to hospital discharge, the 110 (22 %) who died in the subsequent year were older (P < 0.001) and more likely to have been discharged to a nursing home, other hospital, or hospice compared to patients alive at 1 year (P < 0.001). Important predictors of death among hospital survivors were comorbidities present at the time of ARDS, and not living at home prior to admission. ARDS-related measures of severity of illness did not emerge as independent predictors of mortality in hospital survivors.
Conclusions
Despite improvements in short-term ARDS outcomes, 1-year mortality is high, mostly because of the large burden of comorbidities, which are prevalent in patients with ARDS.
Similar content being viewed by others
Introduction
The last decade has seen many advancements in care for patients with acute respiratory distress syndrome (ARDS) including improvements in ventilator management [1, 2], noninvasive mechanical ventilation strategies [3–5], sepsis management [6], and intensive care unit system changes [7, 8]. More patients with ARDS are now surviving to hospital discharge, a phenomenon that has been reported in both observational studies and randomized trials [9–11]. For example, 60-day mortality decreased from 40 % in the traditional tidal volume arm of the ARDS network ARMA study published in 2000 to 21 % in the most recent ARDS network trial published in 2011, in spite of increased severity of illness and more comorbidities in the more recent trial [1, 12]. However, less is known about the epidemiology of long-term survival in ARDS, particularly in the context of increasing severity of illness and comorbidities among ICU patients today [13].
Initial studies of long-term outcomes in ARDS found that in-hospital survival from ARDS provided a good estimate of long-term survival [14–17]; however, these populations are not reflective of ARDS patients in modern practice. In-hospital mortality in these studies was higher at 37–40 %, as these cohorts predated widespread implementation of low tidal volume ventilation. Furthermore, patients who survived to hospital discharge and were selected for these studies were young (mean age mid-40 s), had few coexisting conditions, and lower severity of illness on presentation. More recent data demonstrate a widening difference between ARDS survival at discharge and long-term follow-up [11, 18, 19]. This “survival gap” suggests that while modern ICU interventions have resulted in short-term improvements in ARDS survival, the overall survival after ARDS may not have improved. Temporal changes in ICU patient characteristics including increased severity of illness and more comorbid illnesses may explain some of the changes in trajectory of illness following discharge [13].
Although factors that predict short-term mortality have been well described [20–29], a better understanding of predictors of long-term mortality in ARDS is required for improved prognostication and a better understanding of the effects of ICU interventions on long-term outcomes [19]. A small study predating low tidal volume ventilation found that comorbidities, ARDS risk factor, and age were most highly associated with mortality 6 months following ARDS diagnosis [30], but the influence of increased severity of illness over time has not been assessed. A more recent study found that the survival benefits of adherence to low tidal volume ventilation persisted at 2 years follow-up, suggesting that modern changes in clinical practice may provide long-standing improvements in survival [19]. However, this study excluded sicker patients with cancer or life-limiting disease—diagnoses that are frequent in patients presenting with ARDS. The predictors and causes of death in a broad sample of patients surviving to hospital discharge with ARDS are not known.
The purpose of the current study was to (1) quantify the gap between in-hospital and 1-year ARDS mortality in modern practice, (2) identify the most influential risk factors for death at 1 year among hospital survivors in a large, multi-ICU prospective cohort study of patients with ARDS, and (3) examine causes of death among patients dying in the year after diagnosis of ARDS. Some of these data have previously been presented in the form of an abstract [31].
Materials and methods
Subjects
The study subjects were drawn from the validation of biomarkers in acute lung injury diagnosis (VALID) study, an ongoing prospective, multi-ICU cohort study of critically ill patients at Vanderbilt University Medical Center, a tertiary medical center in Nashville, TN. The inclusion criteria and exclusion criteria of VALID have been reported previously and are described in the supplemental appendix [32]. Specifically, patients with severe chronic lung disease were excluded, but patients with other underlying comorbidities including advanced cancer and HIV were not excluded. The study was approved by the Vanderbilt University Institutional Review Board.
For this analysis, we included patients enrolled between January 2006 and February 2010 who had or developed acute lung injury (ALI)/ARDS during the first 4 days after admission to the ICU. During the study period, there were a total of 2,181 total patients enrolled in VALID of whom, 1,894 had at least one risk factor for ARDS. Of these, the current study focused on the 646 who met the American European consensus conference (AECC) criteria for ALI/ARDS [33].
Data collection
Data collection and definitions have been described previously [32]. Diagnosis of ALI/ARDS was defined by the AECC definition (PaO2/FiO2 ≤300 mmHg for ALI and PaO2/FiO2 ≤200 mmHg for ARDS) [33], and could be established at any time during the first 4 days in the ICU. Both mechanically ventilated (defined as invasive mechanical ventilation within 4 days of AECC ALI/ARDS diagnosis) and not mechanically ventilated patients meeting AECC criteria were included in our primary analysis. In a sensitivity analysis, we also considered the Berlin definition for ARDS [34, 35]. Berlin severity levels were defined as mild (200 mmHg <PaO2/FiO2 ≤300 mmHg with PEEP or CPAP ≥5 cmH2O), moderate (100 mmHg <PaO2/FiO2 ≤200 mmHg with PEEP ≥5 cmH2O), and severe (PaO2/FiO2 ≤100 mmHg with PEEP ≥5 cmH2O). For both AECC and Berlin definitions, the ratio of pulse oximetric saturation to fraction of inspired oxygen (SpO2/FiO2) was used as a validated surrogate for PaO2/FiO2 among patients without an arterial blood gas measurement at the time of ALI/ARDS diagnosis: SpO2/FiO2 = 64 + 0.84 × (PaO2/FiO2) [36]. The discharge location for patients who survived hospitalization was categorized as home, rehabilitation hospital, nursing home, hospice, or other acute care hospital. The lung injury score [37], Brussels organ failure [38], and definitions for sepsis, pneumonia, aspiration, and trauma are included in the supplemental appendix.
Outcome measures
All patients were followed until death or for at least 1 year after study enrollment. Short-term mortality was defined as all-cause mortality during hospitalization. Long-term mortality was defined as all-cause mortality 1 year following enrollment in VALID in patients who survived to hospital discharge. Patient deaths were identified by medical record review and query of the social security death index (SSDI) [39]. Causes of death were determined by review of death certificate data obtained from the Tennessee Vital Records Office of the Tennessee Department of Health. Underlying causes of death were categorized as infection, malignancy (primary, metastatic, or hematologic), cardiovascular, respiratory, gastrointestinal/hepatic, trauma, or other causes (listed in supplemental appendix) based on ICD-9 coding of the primary cause of death on the death certificate.
Statistical analysis
We used t tests, chi-squared tests, and Fisher’s exact tests as appropriate to compare baseline demographics and clinical risk factors across groups. Descriptive statistics and McNemar’s were used to compare in-hospital and 1-year mortality. Since all events were accounted for over the year of follow-up, logistic regression was used to analyze associations between candidate risk factors and mortality. Kaplan–Meier survival plots demonstrate the time from discharge to death in hospital survivors and the log-rank test was used to estimate differences according to discharge location. Clinical characteristics associated with 1-year mortality with P < 0.10 on bivariable analysis were entered into a stepwise elimination model to retain potential risk factors if they remained associated at a P value of less than 0.05. Because of the modest number of outcomes, the forward stepwise elimination was used to maintain model parsimony. In a sensitivity analysis, we analyzed only patients meeting the Berlin definition of ARDS [34]. Analyses were performed using STATA version 10 (STATA Corp, College Station, TX). Statistical significance was defined as a two-tailed P < 0.05 for all analyses.
Results
Clinical characteristics and outcomes
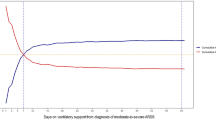
Among 646 patients with ALI/ARDS, the proportion of patients who died increased from 24 % (n = 153, 95 % CI 21–27 %) during hospitalization to 41 % (n = 263, 95 % CI 37–45 %) during the year after discharge (P < 0.0001). One-year mortality was higher than in-hospital mortality regardless of ALI/ARDS etiology (Supplemental Fig. 1). In the subset of patients with 2-year outcomes available (n = 527, 82 %), the 2-year cumulative incidence of death was 54 % (n = 282, 95 % CI 49–59 %, P = 0.0004).
In a sensitivity analysis of 551 patients meeting the Berlin definition of ARDS [34] (excluded 95 patients: 87 non-mechanically ventilated in first 4 days of enrollment; two patients with missing PEEP; two patients with PEEP <5 cmH2O; and four patients not meeting hypoxemia criteria on day otherwise meeting all Berlin criteria) we found similar rates of hospital and 1-year mortality (Supplemental Table 1). Severity of ARDS defined by Berlin levels (mild, moderate, severe) was associated with in-hospital mortality but not with mortality at 1-year among hospital survivors.
Comparison of baseline characteristics by hospital and 1-year outcomes
Demographics, comorbidities, and initial clinical characteristics did not differ substantially between those who died early (in hospital) and those who died over the subsequent year (Table 1). Patients who died in the hospital (n = 153) were more likely to have a hematologic malignancy and less likely to have COPD or metastatic cancer than patients who died after surviving hospitalization but were otherwise demographically similar. In addition, there was no difference in underlying cause of ALI/ARDS although patients who died during hospitalization had a lower P/F ratio and a higher incidence of hepatic failure compared to those dying after hospitalization.
By contrast, compared to patients who died in the year following hospital discharge (n = 110), survivors at 1 year (n = 383) were younger, were more likely to have been admitted through the emergency department and had substantially fewer comorbidities such as COPD, HIV, diabetes, chronic heart failure, chronic kidney disease, or malignancy (Table 1). In addition, patients who were alive at 1 year were more likely to have trauma and less likely to have sepsis as the cause of ALI/ARDS. Increased severity of illness on presentation was associated with higher 1-year mortality among patients who survived hospitalization: respiratory rate, APACHE II score, presence of coagulation failure, renal failure, and circulatory failure were all significantly associated with death after discharge (Table 1).
Comparison of hospital course between hospital survivors who were dead or alive at 1 year
Among patients with ALI/ARDS who survived hospitalization, those who survived to 1 year had significantly shorter time from hospital admission to ICU admission, lower creatinine at discharge, and were more likely to be discharged home or to a rehabilitation facility and less likely to be discharged to a nursing home or hospice facility (Table 2). Specifically, discharge destination among hospital survivors was strongly associated with long-term mortality (Fig. 1) (P < 0.001). There were no differences in ICU length of stay (P = 0.76) or duration of mechanical ventilation (P = 0.62) between hospital survivors that died and survived at 1-year follow-up.
Probability of survival to 1 year of follow-up among hospital survivors according to discharge location. Whereas 15 % (34/230) and 13 % (15/115) of patients discharged to home or rehabilitation died in the year of follow-up, respectively, 25 % (19/76) transferred to another hospital, 38 % (19/50) discharged to a nursing home, and 96 % (22/23) discharged to hospice care died. The differences in survival across groups are statistically significant (P < 0.001) driven by significantly increased 1-year mortality among patients discharged to hospice (P < 0.001), nursing home (P < 0.001), and other hospital (P = 0.03) compared to discharge to home
Independent predictors of 1-year mortality after discharge
Stepwise elimination identified several baseline characteristics as independent predictors of mortality among hospital survivors. These included age and severe comorbidities: HIV, metastatic cancer, hematologic malignancy, non-metastatic cancer, and chronic renal disease (Table 3). Trauma as a cause of ARDS and living at home prior to hospitalization were strong independent predictors of decreased odds of mortality at 1 year. Increased length of hospital stay was the only characteristic of hospitalization that was independently associated with increased odds of death at 1 year among hospital survivors. Severity of illness measures including LIS, APACHE II, organ failure, and PaO2/FiO2 did not emerge as independent predictors of mortality in survivors. The C-statistic for the final adjusted model was 0.81. Predictors of 1-year mortality among survivors were similar after excluding 23 patients discharged to hospice, and characteristics associated with 1-year mortality among those surviving the hospitalization were similar to those associated with overall 1-year mortality (data not shown).
Causes of death
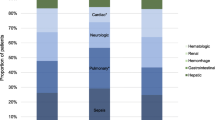
Death certificate data was available for 244 (93 %) of the 263 patients who died within 1 year of enrollment. Overall, the most common underlying cause of death both in the hospital and among hospital survivors was malignancy (Fig. 2). Seventy-two percent of patients with known malignancy at the time of ARDS were found to have malignancy as the underlying cause of death at 1 year. There were no significant differences in underlying cause of death between patients who died in the hospital and patients who died after discharge.
Underlying causes of death in patients with ALI/ARDS who died during the hospital stay (gray bars) and those who survived hospitalization but died during the year following enrollment (black bars). In both groups, the most common cause of death was underlying malignancy. No difference is statistically significant
Discussion
Short-term mortality in ARDS has declined over the last decade owing to improvements in supportive care and of the use of protective ventilator strategies [9, 10]. We sought to quantify the survival gap between short- and long-term ARDS mortality and identify risk factors for death and causes of death at 1 year for hospital survivors. In this study of a broad, heterogeneous cohort of critically ill patients with ALI/ARDS, overall hospital mortality was 24 %, concordant with short-term mortality rates of ALI/ARDS mortality reported in the era of low tidal volume ventilation [10, 11], and 1-year mortality was substantially higher at 41 %, consistent with other recent studies [11, 18, 19]. This finding did not vary according to etiology of ARDS or in the presence of sepsis. Furthermore, in a large subgroup of patients followed for 2 years, we found that more than half of the patients with ALI/ARDS had died. Disposition after hospitalization was highly associated with 1-year mortality, suggesting that functional status after discharge may be an important contributing factor to long-term mortality after ARDS. The independent predictors of death at 1 year were age, living somewhere other than home prior to admission, and serious comorbidities. Although several measures of severity of illness and characteristics of hospital course were associated with long-term mortality among hospital survivors, only length of hospital stay remained an independent predictor of long-term mortality in a stepwise elimination model. Restriction of the analysis to patients meeting the Berlin definition for ARDS did not change the findings, and Berlin level of severity of ARDS did not predict long-term mortality in hospital survivors.
This study provides several insights into recent reports of declines in ARDS mortality. Although short-term mortality in ARDS has decreased in the last decade, our findings expand upon other recent studies demonstrating a widening survival gap between ARDS survival at discharge and long-term follow-up [11, 18, 19]. This gap suggests that while modern ICU interventions have improved short-term outcomes in ARDS, other factors contribute to a persistently high long-term mortality in ARDS. One possible explanation is the temporal changes in characteristics of ICU patients over the last several decades. Large studies have demonstrated that older age, the number of comorbidities, and the severity of illness have increased both in the general ICU population and in ARDS patients specifically [10, 13]. ICU treatment cannot address the underlying comorbidities and increasing age that ultimately contribute to high long-term mortality in ICU patients today. Although a recent study found that adherence to low tidal volume ventilation in ARDS was associated with a survival benefit up to 2 years following hospitalization, these conclusions may only be generalizable to the most healthy of ARDS patients because those with significant comorbidities, poor social status, and life-limiting illnesses were excluded from enrollment in the observational study [19].
Our findings are consistent with prior studies focusing on long-term mortality in general critically ill patients, a finding that supports the hypothesis that long-term outcomes in ARDS are more related to the medical conditions and age of patients requiring ICU care in general rather than the development of ARDS specifically [30]. In a recent study of Medicare patients, ICU survivors had higher 3-year mortality than non-ICU hospital survivors or non-hospitalized controls, and a separate study showed that critically ill patients have decreased survival for up to 15 years compared to age- and sex-matched population controls [40, 41]. Furthermore, predictors of long-term outcomes for critically ill patients are similar to those we observed in ARDS, with comorbidities as top predictors for subsequent re-hospitalization and death [41]. One possible explanation for the persistently high risk of death in patients with critical illness includes a persistent pro-inflammatory state that may exacerbate or trigger other underlying inflammatory disorders including cardiovascular disease, recurrent infection, cancer, and renal failure—all common causes of death among patients in our study who survived to hospital discharge.
This study has several strengths including the large sample size, broad patient population with few exclusion criteria, and the careful prospective phenotyping for ALI/ARDS, sepsis, and other important clinical variables. Because there were very few exclusion criteria for enrollment in VALID, the findings are likely to be generalizable. The study also has some limitations. First, it is a single-center study. However, this is counterbalanced by the broad spectrum of heterogeneous critically ill patients from four different intensive care units included. Second, it is possible that we underestimated the 1-year mortality rate of ARDS survivors; although the SSDI has been shown to be a valuable tool for determining long-term outcomes [39], some patients without social security numbers may not be included. Third, mortality does not capture the full burden of disease. Long-term sequelae of ARDS, including impaired pulmonary function, neuromuscular weakness, and neuropsychiatric are well described [15, 42–45]. However, these limitations would have led us to underestimate rather than overestimate long-term effects of ARDS and do not weaken the findings. Finally, we were unable to test the effect of specific ICU interventions such as low-tidal volume ventilation on long-term outcomes.
Conclusions
Long-term mortality is substantially higher than short-term mortality in a broad sample of patients with ARDS. In spite of improvements in supportive care and significantly improved short-term outcomes, long-term outcomes remain poor. The top predictors of 1-year mortality in hospital survivors include non-modifiable factors including age and comorbidities, and the most common causes of death are malignancy and infection. These results underscore the importance of considering the interaction between comorbid illness and ARDS on the trajectory of long-term outcomes in hospital survivors of ARDS for testing new interventions and providing prognoses. Researchers must measure whether effects of interventions can influence the overall trajectory of survival in ARDS patients who do and do not have major comorbidities. Possible clinical applications of such research include improved guidance of initial discussions of prognosis and the benefits of full resuscitation for high-risk patients. For clinical trials, it is perhaps more realistic to target shorter-term endpoints such as 30- to 90-day mortality, since 1-year mortality will be driven primarily by comorbidities that cannot be reversed or influenced by treatments for increasing survival from ARDS such as lung protective or prone ventilation. This study, along with others, demonstrates that premorbid illnesses are the top predictors of long-term outcomes after ARDS in critically ill patients today.
References
The Acute Respiratory Distress Syndrome Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308
Sinha P, Flower O, Soni N (2011) Deadspace ventilation: a waste of breath! Intensive Care Med 37:735–746
Antonelli M, Conti G, Esquinas A, Montini L, Maggiore SM, Bello G, Rocco M, Maviglia R, Pennisi MA, Gonzalez-Diaz G, Meduri GU (2007) A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med 35:18–25
Carrillo A, Gonzalez-Diaz G, Ferrer M, Martinez-Quintana ME, Lopez-Martinez A, Llamas N, Alcazar M, Torres A (2012) Non-invasive ventilation in community-acquired pneumonia and severe acute respiratory failure. Intensive Care Med 38:458–466
Piquilloud L, Tassaux D, Bialais E, Lambermont B, Sottiaux T, Roeseler J, Laterre PF, Jolliet P, Revelly JP (2012) Neurally adjusted ventilatory assist (NAVA) improves patient-ventilator interaction during non-invasive ventilation delivered by face mask. Intensive Care Med 38:1624–1631
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377
Pronovost PJ, Rinke ML, Emery K, Dennison C, Blackledge C, Berenholtz SM (2004) Interventions to reduce mortality among patients treated in intensive care units. J Crit Care 19:158–164
Valentin A, Ferdinande P, Improvement EWGoQ (2011) Recommendations on basic requirements for intensive care units: structural and organizational aspects. Intensive Care Med 37:1575–1587
Zambon M, Vincent JL (2008) Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest 133:1120–1127
Erickson SE, Martin GS, Davis JL, Matthay MA, Eisner MD (2009) Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med 37:1574–1579
Sigurdsson MI, Sigvaldason K, Gunnarsson TS, Moller A, Sigurdsson GH (2013) Acute respiratory distress syndrome: nationwide changes in incidence, treatment and mortality over 23 years. Acta Anaesthesiol Scand 57:37–45
Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, Hite D, Holets S, Kallet RH, Liu KD, Macintyre N, Moss M, Schoenfeld D, Steingrub J, Thompson BT (2011) Randomized, placebo-controlled clinical trial of an aerosolized beta-2 agonist for treatment of acute lung injury. Am J Respir Crit Care Med 184:561–568
Zimmerman JE, Kramer AA, Knaus WA (2013) Changes in hospital mortality for United States intensive care unit admissions from 1988 to 2012. Crit Care 17:R81
Angus DC, Musthafa AA, Clermont G, Griffin MF, Linde-Zwirble WT, Dremsizov TT, Pinsky MR (2001) Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med 163:1389–1394
Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS (2003) One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 348:683–693
Cheung AM, Tansey CM, Tomlinson G, Diaz-Granados N, Matte A, Barr A, Mehta S, Mazer CD, Guest CB, Stewart TE, Al-Saidi F, Cooper AB, Cook D, Slutsky AS, Herridge MS (2006) Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med 174:538–544
Heyland DK, Groll D, Caeser M (2005) Survivors of acute respiratory distress syndrome: relationship between pulmonary dysfunction and long-term health-related quality of life. Crit Care Med 33:1549–1556
Clermont G, Kong L, Weissfeld LA, Lave JR, Rubenfeld GD, Roberts MS, Connors AF Jr, Bernard GR, Thompson BT, Wheeler AP, Angus DC, Network NACT (2011) The effect of pulmonary artery catheter use on costs and long-term outcomes of acute lung injury. PLoS One 6:e22512
Needham DM, Colantuoni E, Mendez-Tellez PA, Dinglas VD, Sevransky JE, Dennison Himmelfarb CR, Desai SV, Shanholtz C, Brower RG, Pronovost PJ (2012) Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ 344:e2124
Doyle RL, Szaflarski N, Modin GW, Wiener-Kronish JP, Matthay MA (1995) Identification of patients with acute lung injury. Predictors of mortality. Am J Respir Crit Care Med 152:1818–1824
Zilberberg MD, Epstein SK (1998) Acute lung injury in the medical ICU: comorbid conditions, age, etiology, and hospital outcome. Am J Respir Crit Care Med 157:1159–1164
Monchi M, Bellenfant F, Cariou A, Joly LM, Thebert D, Laurent I, Dhainaut JF, Brunet F (1998) Early predictive factors of survival in the acute respiratory distress syndrome. A multivariate analysis. Am J Respir Crit Care Med 158:1076–1081
Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, Matthay MA (2002) Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 346:1281–1286
Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP (2005) Causes and timing of death in patients with ARDS. Chest 128:525–532
Cooke CR, Kahn JM, Caldwell E, Okamoto VN, Heckbert SR, Hudson LD, Rubenfeld GD (2008) Predictors of hospital mortality in a population-based cohort of patients with acute lung injury. Crit Care Med 36:1412–1420
Seeley E, McAuley DF, Eisner M, Miletin M, Matthay MA, Kallet RH (2008) Predictors of mortality in acute lung injury during the era of lung protective ventilation. Thorax 63:994–998
Cooke CR, Shah CV, Gallop R, Bellamy S, Ancukiewicz M, Eisner MD, Lanken PN, Localio AR, Christie JD (2009) A simple clinical predictive index for objective estimates of mortality in acute lung injury. Crit Care Med 37:1913–1920
Brown LM, Calfee CS, Matthay MA, Brower RG, Thompson BT, Checkley W (2011) A simple classification model for hospital mortality in patients with acute lung injury managed with lung protective ventilation. Crit Care Med 39:2645–2651
Villar J, Perez-Mendez L, Blanco J, Anon JM, Blanch L, Belda J, Santos-Bouza A, Fernandez RL, Kacmarek RM, Spanish Initiative for Epidemiology S, Therapies for AN (2013) A universal definition of ARDS: the PaO2/FiO2 ratio under a standard ventilatory setting—a prospective, multicenter validation study. Intensive Care Med 39:583–592
Davidson TA, Rubenfeld GD, Caldwell ES, Hudson LD, Steinberg KP (1999) The effect of acute respiratory distress syndrome on long-term survival. Am J Respir Crit Care Med 160:1838–1842
Wang CY, Kangelaris KN, Janz, May AK, Z H, Bernard GR, Matthay MA, Calfee CS, Ware LB (2012) Long term mortality in clinical acute lung injury is dramatically higher than hospital mortality (abstract). Am J Respir Crit Care Med 102:A102 A2297–A2297
Siew ED, Ware LB, Gebretsadik T, Shintani A, Moons KG, Wickersham N, Bossert F, Ikizler TA (2009) Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol 20:1823–1832
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R (1994) The American–European consensus conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149:818–824
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS (2012) Acute respiratory distress syndrome: the Berlin definition. JAMA 307:2526–2533
Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, Brochard L, Brower R, Esteban A, Gattinoni L, Rhodes A, Slutsky AS, Vincent JL, Rubenfeld GD, Thompson BT, Ranieri VM (2012) The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 38:1573–1582
Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB (2007) Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest 132:410–417
Murray JF, Matthay MA, Luce JM, Flick MR (1988) An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 138:720–723
Bernard GR, Wheeler AP, Arons MM, Morris PE, Paz HL, Russell JA, Wright PE (1997) A trial of antioxidants N-acetylcysteine and procysteine in ARDS. The antioxidant in ARDS study group. Chest 112:164–172
Quinn J, Kramer N, McDermott D (2008) Validation of the social security death index (SSDI): an important readily-available outcomes database for researchers. West J Emerg Med 9:6–8
Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT (2010) Three-year outcomes for medicare beneficiaries who survive intensive care. JAMA 303:849–856
Williams TA, Dobb GJ, Finn JC, Knuiman MW, Geelhoed E, Lee KY, Webb SA (2008) Determinants of long-term survival after intensive care. Crit Care Med 36:1523–1530
Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson LV (1999) Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med 160:50–56
Unroe M, Kahn JM, Carson SS, Govert JA, Martinu T, Sathy SJ, Clay AS, Chia J, Gray A, Tulsky JA, Cox CE (2010) One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann Intern Med 153:167–175
Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, Kudlow P, Cook D, Slutsky AS, Cheung AM, Canadian Critical Care Trials Group (2011) Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 364:1293–1304
Chiumello D, Taccone P, Berto V, Marino A, Migliara G, Lazzerini M, Gattinoni L (2012) Long-term outcomes in survivors of acute respiratory distress syndrome ventilated in supine or prone position. Intensive Care Med 38:221–229
Acknowledgments
We thank all the patients who participated in the study and the research staff who assisted with the study. We are grateful to Dr. David Law and Mr. John Brown of the Tennessee Department of Health for assistance with obtaining death certificate data for this study. An abstract of these findings was presented at the American Thoracic Society International Conference in May 2012. At the time the research was conducted Dr. Wang was supported by MCRR/NIH UL1 RR024975-01. Dr. Kangelaris was supported by the Society of Hospital Medicine Young Researchers Award, the NIH National Center for Advancing Translational Sciences through UCSF-CTSI KL2 TR000143, and NHLBI 1K23HL116800-01. Dr. Calfee was supported by NHLBI HL090833 and HL110969. Dr Ware was supported by NHLBI HL081332, and HL103836 and the American Heart Association Established Investigator Award. Dr. Matthay was supported by NHLBI HL51856.
Conflicts of interest
No author reports a conflict of interest. Dr. Calfee has served on medical advisory boards for Cerus Corp and GlaxoSmithKline.
Author information
Authors and Affiliations
Corresponding author
Additional information
Take-home message: This prospective cohort study indicates that in-hospital mortality substantially underestimates mortality at 1 year following ARDS. The independent risk factors for death in hospital survivors of ARDS were age and comorbidities rather than ARDS-related severity of illness measures. These findings highlight the prevalence of serious comorbidities among ARDS patients in the modern era and suggest that underlying comorbidities are most predictive of the long-term trajectory of ARDS patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, C.Y., Calfee, C.S., Paul, D.W. et al. One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome. Intensive Care Med 40, 388–396 (2014). https://doi.org/10.1007/s00134-013-3186-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-013-3186-3