Abstract
Neural respiratory drive, i.e., the activity of respiratory centres controlling breathing, is an overlooked physiologic variable which affects the pathophysiology and the clinical outcome of acute respiratory distress syndrome (ARDS). Spontaneous breathing may offer multiple physiologic benefits in these patients, including decreased need for sedation, preserved diaphragm activity and improved cardiovascular function. However, excessive effort to breathe due to high respiratory drive may lead to patient self-inflicted lung injury (P-SILI), even in the absence of mechanical ventilation. In the present review, we focus on the physiological and clinical implications of control of respiratory drive in ARDS patients. We summarize the main determinants of neural respiratory drive and the mechanisms involved in its potentiation, in health and ARDS. We also describe potential and pitfalls of the available bedside methods for drive assessment and explore classical and more “futuristic” interventions to control drive in ARDS patients.
Similar content being viewed by others
Determinants of respiratory drive
Healthy subjects
Breathing is generated by the rhythmic discharge of groups of neurons located in the brainstem which produces a neural signal directed to respiratory muscles to generate inspiratory effort and tidal breathing [1, 2]. In humans, the activity of the respiratory centres requires a tonic excitatory input that derives from two sources: a chemosensory or “automatic” input and a descending or “behavioural” input.
The chemosensory input is a feedback reflex mediated by afferents from central and peripheral chemoreceptors aimed at minimizing fluctuations of the partial pressure of arterial carbon dioxide (PaCO2) and pH and correcting hypoxemia. Central chemoreceptors, located in the ventral surface of the medulla oblongata, regulate the ventilatory response to stabilize CO2: an increase in PaCO2, by decreasing the pH of cerebrospinal fluid, leads to a linear increase in minute ventilation until steady-state is achieved after a few minutes [3]. The peripheral chemoreceptors, located in the carotid bodies, stimulate breathing by modifying the sensitivity and threshold of the central chemoreceptors, specifically providing faster and more intense responses to modifications in PaCO2 and pH and to hypoxemia [3,4,5].
The descending input is a feed-forward pathway from cortical brain centres and is responsible for adaptive changes of breathing pattern during complex activities, such as physical exercise and mental tasks [6, 7]. Both the chemosensory and the central input are active in awake healthy subjects [8, 9]. Indeed, artificially induced hypocapnia (e.g., through mechanical ventilation) does not abolish respiratory drive [9]. In addition, the respiratory rhythm is modulated by signals from the limbic system, which alters the breathing pattern in response to cognitive and emotional factors, including pain and anxiety [10].
In physiological studies, the response of the subject to raised PaCO2 level is assessed by measuring the increase in minute ventilation. In this context, two curves exist: the “brain curve”, that describes the minute ventilation requested by the neural respiratory drive for a given PaCO2; and the “ventilation curve”, that describes the actual minute ventilation of the subject for a given PaCO2. In health, the brain curve coincides with the ventilation curve. The levels of PaCO2 and the corresponding minute ventilation show a linear correlation, the slope of which represents the “brain” respiratory drive [11]. The actual point of equilibrium will lie at the intersection between this neural drive and the metabolic hyperbola, which, instead, is the relationship between ventilation and the resultant PaCO2 for a given level of metabolic CO2 production and dead space [11, 12] (Fig. 1a).
Metabolic hyperbola, brain and ventilation curves in health and ARDS. The metabolic hyperbola is the relationship between ventilation and the resultant PaCO2 for a given level of metabolic CO2 production and dead space. Increased dead space or CO2 production will shift the hyperbola up. The ventilation curve describes the actual effect of changing PaCO2 on resultant minute ventilation. ARDS can shift the ventilation curve to the right (lower minute ventilation despite higher PaCO2) due to increased respiratory load and muscle weakness. Finally, the brain curve (also known as the "controller curve", "CO2 sensitivity curve" or "ventilation gain curve") describes the minute ventilation theoretically requested by the neural respiratory drive for a given PaCO2. During ARDS, this is shifted to the left (higher minute ventilation despite lower PaCO2) due to multiple concomitant pathologic conditions, including acidosis, inflammation and others. a In health, brain and ventilation curves overlap and the ventilation response (i.e., the change in minute ventilation induced by a change in PaCO2) reflects the neural respiratory drive. The metabolic hyperbola is obtained assuming a dead space of 0.3 and a metabolic CO2 production (VCO2) of 200 ml/min. Brain and ventilation curves are overlapping and are calculated assuming at PaCO2 of 39.5 mmHg, a ventilation of 6.5 l/min, linearly increasing to 30 l/min at a PaCO2 of 49 mmHg. b In ARDS, the metabolic hyperbola is shifted upward due to increase of dead space (0.5) and VCO2 (250 ml/min). The listed factors cause the brain and ventilation curves to be shifted in opposite directions and diverge. Please, note that a single ARDS patient will be characterized by both curves at the same time: the brain curve will correspond to the theoretical ventilation/PaCO2 correlation desired by the neural respiratory drive, while the ventilation curve will be the actual ventilation/PaCO2 correlation measured by spirometer and blood gas analysis. Brain and ventilation curves are calculated assuming a ventilation of 6.5 l/min at 28 mmHg PaCO2 (increasing to 30 l/min at 33 mmHg PaCO2) and a ventilation of 5 l/min at 40 mmHg PaCO2 (increasing to 25 l/min at 52 mmHg PaCO2), respectively
Acute respiratory distress syndrome
The brain curve, the ventilation curve and the metabolic hyperbola are all potentially modified in ARDS: increased dead space and metabolic CO2 production shift the metabolic hyperbola upward, meaning that PaCO2 is higher than normal for a given minute ventilation [11]; the slopes and the position of the brain curve and the ventilation curve are altered in opposite directions (Fig. 1b).
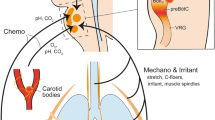
In ARDS, pulmonary interstitial and alveolar edema result in increased intra-pulmonary shunt and dead space, and decreased functional lung size due to alveolar collapse (the so-called “baby lung”) [13]. Systemic inflammation is common and extra-pulmonary organ dysfunction frequently develops (Fig. 2).
Schematic representation of control of respiratory drive in ARDS. The figure shows the key triggers of respiratory drive and the anatomic targets where these triggers exert their effects. In the centre, the descending cascade from neural respiratory drive to breathing effort and lung stress is represented, together with the main factors that may cause a dissociation between drive and effort (i.e., muscle function) and between drive, effort and lung stress (i.e., neuromechanical coupling and respiratory mechanics)
Impairment of gas exchange leads to an increase in chemosensory input. Increase in PaCO2, promoted by high dead space, induces a linear increase in respiratory drive through both central and peripheral chemoreceptors [14, 15]. On the other hand, the ventilatory response following severe hypoxemia typical of ARDS is not linear but hyperbolic [11]. Peripheral chemoreceptors, which are relatively insensitive at mild hypoxemia, increase the neural respiratory drive in response to more severe hypoxemia, mainly by enhancing the ventilatory response to CO2 when the partial pressure of arterial oxygen (PaO2) falls below 60–70 mmHg. This effect can be potentiated by concomitant hypercapnia. Metabolic acidosis, which frequently complicates ARDS because of shock or acute kidney injury, stimulates both peripheral and central chemoreceptors [16].
In addition, ARDS might be associated with alterations of neural respiratory drive induced by mechanisms specifically associated with lung inflammation and altered mechanics. In awake spontaneously breathing rats, ARDS induces an increase in respiratory rate occurring before impairment of gas exchange. Hypoxic ventilatory response is also exaggerated due to a sensitization of peripheral chemoreceptors [17]. Local and systemic inflammation are hallmarks of ARDS [18], and pulmonary C-fibers sensitive to inflammatory mediators (including histamine, bradykinin and prostaglandins) are consistently activated in lung edema [19] and experimental acute lung injury [20]. Vagal afferents from these lung chemoreceptors can modulate the breathing pattern through a central reflex pathway [21]. The consequence of vagal activation is an increase in respiratory rate with a decrease in tidal volume, i.e., rapid shallow breathing [22, 23], possibly through vagally mediated release of cytokines in the brainstem [24].
The lung also contains mechanoreceptors: slowly adapting receptors (SARs) are stretch receptors activated by lung inflation that inhibit central chemoreceptors in rats (for example during the Hering-Breuer reflex), terminating inspiration. Although the Hering-Breuer reflex might be inhibited by the behavioural control of breathing in awake humans [25], decreased inhibitory input from these mechanoreceptors in the atelectatic lung could promote a further increase in inspiratory effort in ARDS. Indeed, the activation of mechanoreceptors appears to decrease as ARDS develops [20], while it is increased by increasing positive end-expiratory pressure (PEEP) [26], probably through stabilized lung recruitment. This could be one of the mechanisms by which high PEEP decreases spontaneous respiratory effort in ARDS [27]. Stimulation of the respiratory centres through each of these mechanisms increases the slope—and shift to the left—of the brain curve. Decreases in lung and chest wall compliance increase the elastic load and can alter the neuro-mechanical coupling between effort and diaphragmatic excursion. The result is a decreased slope of the ventilation curve and an increase in PaCO2, which induces a stimulation of neural respiratory drive and a dissociation between brain and ventilation curves (Fig. 3).
Potential dissociation between neural respiratory drive (P0.1) and respiratory effort (Pes) under pathologic conditions. The figure shows simulated identical waveforms for airway pressure (Paw) during supported breaths but with different simulated oesophageal pressure (Pes) waveforms. P0.1 (i.e., the negative airway pressure generated by occlusion occurring during the first 0.1 s of an inspiration) reflects the intensity of neural respiratory drive. Oesophageal pressure swings (ΔPes) allow quantification of respiratory effort. However, in patients with high chest wall elastance, ΔPes underestimates effort. In the presence of muscular weakness, high drive may be associated with “normal” or even low effort (right panel)
Pain, anxiety and discomfort are common in ARDS patients and all can influence drive. Emotional responses may affect the brain curve independent of a patient’s metabolic demands: anxiety and fear act through the forebrain, limbic and cortical structures and the hypothalamus, processing information from the external environment and directly stimulating spinal respiratory motor neurons [10, 28]. Pain affects the respiratory drive through both behavioural responses and a direct reflex on medullary respiratory centres [28]. On the other hand, the use of sedatives might decrease the neural respiratory drive [29].
Poor patient–ventilator interaction is another determinant of drive in subjects with ARDS on mechanical ventilation. Dyssynchronies might increase the respiratory drive because they cause discomfort and increased respiratory load [30]. Mismatch between the timing and duration of mechanical inflation and the neural inspiratory time prevents effective unloading of the respiratory muscles during assisted ventilation. Moreover, air trapping, which may occur during protective ventilation in ARDS due to the high respiratory rate, could cause additional inspiratory load and delayed trigger, both of which can increase drive.
Of note, the more severe the lung injury, the higher the inspiratory effort reflecting increased activation of neural respiratory drive [31].
How to assess respiratory drive at the bedside
A fundamental difference between ARDS patients and healthy subjects is that ventilatory response may not (and usually does not) mirror the respiratory drive [11]. The alterations in neuromuscular function (muscle weakness) and respiratory mechanics (atelectasis and increased lung and chest wall elastance) generate a discrepancy between the activity of the respiratory centres and the resulting motor output. When the intensity of the signal from the brain to the muscles and to the lung is dampened by these alterations, the force of contraction of respiratory muscles and the changes in intrathoracic pressure, flow and volume underestimate the neural drive. Therefore, clinical surrogates of respiratory drive may be conveniently categorized according to their “distance” from the respiratory centres (Table 1). First, neural output (i.e., electrical activity of the diaphragm); second, breathing effort, assessed by changes in pressure induced by the respiratory muscles (i.e., swings in pleural pressure or P0.1); and, third, ventilatory response, reflected by the tidal volume and respiratory rate (breathing pattern).
Neural output
The electrical activity of the crural diaphragm (EAdi) reflects the phrenic nerve activity and hence the neural output of the respiratory centres to the diaphragm, provided that neuromuscular transmission and muscle excitability are intact. Eadi may be recorded using an oesophageal catheter with multiple electrodes and it represents the “closest” surrogate of neural respiratory drive available in clinical practice [32]. Because of high inter-individual variability, it is difficult to provide references for absolute values of EAdi [33]. However, trends in EAdi allow the tracking of changes in neural output in individual patients [34]. EAdi is also increased in the presence of low muscle strength [35] and the ratio of actual EAdi to maximum EAdi measured during an occlusion may provide an accurate estimate of the patient neural respiratory drive and effort to breathe [33]. The ratio between tidal volume (Vt) and EAdi represents the neuroventilatory efficiency of the diaphragm [36]: a low Vt/EAdi ratio, either due to diaphragm dysfunction or to compromised respiratory mechanics, indicates dissociation between neural respiratory drive and ventilatory response. EAdi monitoring only assesses the activity of the diaphragm. However, recruitment of accessory inspiratory [37] and expiratory [38, 39] muscles is a strong indicator of increased neural respiratory drive due to a mismatch between the respiratory load and the muscle capacity with decreased expiratory time. Surface electromyography of extra-diaphragmatic respiratory muscles could, therefore, integrate the EAdi for a complete assessment of neural respiratory drive [40].
Breathing effort
Indices based on the pressure developed by the respiratory muscles, such as oesophageal pressure swings (ΔPes) and respiratory muscle pressure (Pmus), allow reliable quantification of inspiratory effort determined by the neural respiratory drive [41]. Even though ΔPes at increasing PEEP levels did not correlate with changes in the electrical activity of the diaphragm in ARDS patients in one study [27], driving transpulmonary pressure during active inspiration largely depends on ΔPes in presence of high respiratory effort and could be quite difficult to predict when monitoring only the airway pressure [42]. The pressure generated by the respiratory muscles (Pmus) is computed as the difference between the static recoil pressure of the chest wall and ΔPes. Pmus values higher than 10 cmH2O might indicate high effort [43]. The negative airway pressure generated by occlusion occurring during the first 0.1 s of an inspiration, known as P0.1, is commonly used as an index of respiratory drive [44]. In healthy subjects, P0.1 varies between 0.5 and 1.5 cmH2O. P0.1 values consistently above 3–4 cmH2O indicate high neural respiratory drive and high work of breathing [45, 46]. P0.1 depends on the integrity of neuromuscular transmission. However, as compared with other indices based on breathing effort, it is not affected by moderate reductions of respiratory muscle strength, therefore, representing a reliable index of respiratory drive even in patients with muscular weakness [47].
Breathing pattern
Interpretation of the breathing pattern as a surrogate for respiratory drive is challenging in ARDS patients. In healthy subjects, increases in ventilatory demand are met by initial increases in Vt with constant inspiratory time (Ti), resulting in high mean inspiratory flow (Vt/Ti), that reflects high drive [48,49,50]. Similarly, high Vt (and high Vt/Ti) in spontaneously breathing patients with ARDS suggest dangerous increases in respiratory drive both during noninvasive [51] and invasive mechanical ventilation [52]. Increased respiratory rate occurs only when respiratory drive is three to four times higher than normal and it is detected by an increased ratio of Ti and total breath duration (Ti/Ttot) [49, 50]. However, decreased respiratory compliance [53] and muscular weakness may limit the increase in Vt in ARDS [54]. Increased neural respiratory drive could then lead to early increases in respiratory rate with decreased Ti [55] and the rapid shallow breathing index (respiratory rate divided by tidal volume) [56] might indicate high drive with unsatisfied ventilatory demand.
Finally, high respiratory drive due to mechanical load or metabolic demand results in a reduction of the physiologic variability of breathing [57].
As a “gold standard” for clinical evaluation of respiratory drive is lacking, multilevel assessment might be the most informative approach. While measurements closer to the brain centres more reliably reflect the neural drive, downstream parameters (namely the amplitude and the rate of changes in lung volume and pressure that result in ventilation) provide information about the magnitude of lung stress generated by spontaneous ventilation, which is the determinant of P-SILI [58]. Dyspnea results from the imbalance between load and muscle capacity or from the imbalance between motor output and lung expansion [59]. The complex neural network involved in dyspnea receives afferent information on the respiratory motor output from the brainstem and the motor cortex [60], as well as multiple sensory feedbacks from the chemoreceptors and the mechanoreceptors of the lung and chest wall [61]. The perception of dyspnea depends on the integration of this motor and sensory information, modulated by emotion [62]. Therefore, bedside assessment of dyspnea could allow estimation of the distance between brain and ventilation curves.
Clinical impact of abnormal respiratory drive in subjects with ARDS
Physiological and clinical consequences of high respiratory drive
Use of partially supported modes of ventilation in ARDS patients could entail the advantage of decreasing sedation, improving hemodynamics and preserving respiratory muscle function. However, indications for preserving or restoring spontaneous breathing in patients with ARDS are still controversial because, if respiratory drive is not controlled and causes vigorous spontaneous breathing efforts, this worsens lung and diaphragm injury [31, 63, 64].
The mechanisms underlying additional lung injury due to elevated efforts are multiple and complementary. High transpulmonary pressure during inspiration and large tidal volumes determine an increase in lung stress and strain. Patient–ventilator asynchronies due to high inspiratory effort such as double triggering can also lead to high tidal volume [65]. Even in the presence of protective Vt and pressure, regional injury can still occur because of increased local stress in dependent atelectatic lung regions due to the solid-like behaviour of the diseased lung. In addition, decrease of pleural pressure generated by diaphragmatic contraction is larger in the dependent lung regions drawing air from non-dependent regions before ventilator flow reaches the alveoli (i.e., occult pendelluft phenomenon) [66]. Distribution of tidal volume within the lungs is usually more homogenous during spontaneous breathing as compared to controlled ventilation, but too high effort can lead to ventilation heterogeneity with a larger portion of tidal volumes reaching dependent regions. The increased negative pleural pressure during forceful breathing effort also increases transmural vascular pressure, which promotes additional pulmonary oedema due to increased lung perfusion and lower alveolar pressure [67].
Few animal experimental studies show that high inspiratory effort due to excess inspiratory load might induce diaphragm inflammation [68, 69] and promote diaphragm injury [70].
The clinical impact of these mechanisms still needs to be fully defined. From the lung injury point of view, studies on the effects of early use of neuromuscular blocking agents in ARDS are controversial [52] and a few pilot articles reported beneficial effects of preserved spontaneous breathing versus controlled ventilation on lung aeration [39]. As far as diaphragm function is concerned, a small clinical study in critically ill patients reported that high inspiratory effort may lead to increased diaphragm thickness (which might reflect structural injury) and to prolonged mechanical ventilation [71].
Modulating the respiratory drive in the clinical setting
Ideally, control of respiratory drive in ARDS should reduce the dissociation between brain and ventilation curves [11]. High respiratory drive might be considered “appropriate” when the activating stimulus can be corrected by increased ventilatory response. This is the case for hypercapnia and hypoxemia. Increased ventilation is the physiologic response aimed at correcting these alterations. Conversely, several stimuli that increase the activity of respiratory centres in ARDS are not modified by the ventilation feedback. For example, inflammation, pain and anxiety induce an “inappropriate” high respiratory drive. In the case of an appropriate high neural respiratory drive, the treatment should facilitate the ventilatory response (for example by increasing ventilatory support); on the other hand, inappropriate high drive requires a specific treatment (for example medications for anxiolysis). In the context of ARDS, the effects on the lung should always be monitored and high respiratory drive, either appropriate or inappropriate, should be controlled if it results in the generation of excessive lung stress with consequent increased in the risk of P-SILI.
Multiple strategies are available to modulate the respiratory drive and/or effort in ARDS patients, according to the underlying causes and mechanisms of increased drive (Table 2). These include respiratory support modes and settings, medications and non-pharmacologic interventions.
Interventions for control of respiratory drive
Non-invasive respiratory support
Increased respiratory drive is a hallmark of acute respiratory failure from the outset, with the acute onset of dyspnea as the main presenting symptom [58]. The recommended initial management may now include various forms of non-invasive respiratory support: nasal high flow (NHF) [72], continuous positive airway pressure (CPAP) and non-invasive positive pressure ventilation (NIV) [73]. These options can directly modulate the respiratory drive, albeit by different mechanisms, generating relevant clinical consequences (Table 3).
NHF may reduce drive by wash-out of CO2 from upper airways, decreased CO2 production following decreased inspiratory effort, improved oxygenation and improved dynamic lung compliance [74].
CPAP potentially modulates drive by improving oxygenation by means of positive airway pressure, optimised oxygen delivery and improvement of lung mechanics [75].
NIV may decrease respiratory drive by several mechanisms: unloading respiratory muscles from inspiratory effort, which also reduces CO2 production; as well as improving oxygenation and lung mechanics through increases in PEEP [76].
However, these effects may be mitigated by competing physiologic effects. CPAP can lead to CO2 re-breathing and decreased efficiency of CO2 clearance that could diminish the positive effects on respiratory drive. During NIV, patient intolerance or air leaks may result in intermittent mask removal and promote patient–ventilator dyssynchrony, which, in turn, could increase respiratory drive by discomfort and sleep disruption. Finally, NIV unloads the respiratory muscles by applying positive airway pressure during inspiration, which could lead to unchanged or even increased transpulmonary pressure and additional lung injury [77].
Invasive mechanical ventilation
When invasive mechanical ventilation is instituted, there is often an initial phase of deep sedation, which may decrease the respiratory drive and, occasionally, a period of neuromuscular blockade, which eliminates breathing effort. Once assisted breathing is restored, uncontrolled high respiratory drive may resume as well [63]. In this context, the choice of ventilation mode and settings should aim at decreasing the dissociation between the brain and ventilation curves, while limiting risks of additional lung injury. When the ventilatory response corresponds to the neural respiratory drive, controlling drive is crucial to ensure lung protection. On the other hand, in the presence of a large dissociation between the brain and ventilation curves, lung protection could be maintained even in the presence of increased neural respiratory drive; however, adjusting settings to decrease this dissociation could have additional benefits like improving dyspnea and preventing abnormal breathing patterns (e.g., rapid shallow breathing).
The most commonly used assisted ventilation modes are pressure/volume assist control and pressure support. During assist control, higher peak inspiratory flow delivered by pressure-based mode might better match the need of dyspneic ARDS subjects and mitigate drive, but, at the same time, presence of high inspiratory drive could lead to high tidal volumes, which are not lung-protective. On the other hand, volume assist control allows precise control of set tidal volume and driving transpulmonary pressure independent of the patient’s drive, but, high drive can still generate occult pendelluft and regional overdistension [65].
During PSV, simple settings such as the support level, PEEP and FiO2 [78, 79] could influence the respiratory drive. Potential mechanisms of benefit include unloading of the respiratory muscles, improved mechanics and better oxygenation. Oppositely, the drive increases when the ventilator support is reduced. However, unprotective levels of ventilation should not be tolerated in order to comply with the patient respiratory drive during PSV: switching back to controlled ventilation might be safer when inspiratory plateau pressure is higher than 30 cmH2O, Vt greater than 6–8 ml/kg predicted body weight and high levels of FiO2 (e.g., > 80%) are needed [58]. Alternative modes of assisted ventilation with non-fixed support proportional to diaphragm electrical activity [80] or to a desired range of work of breathing performed by the patient [81] are emerging as possibly safer alternatives to increase support without risking excessive additional lung injury. Indeed, during these modes, the drive decreases when support by the ventilator is increased, but, at the same time, the Vt and inspiratory pressure increases only up to a point below safe thresholds, likely because of preserved reflexes limiting lung volumes. Finally, artificially introducing some variability within the respiratory pattern by noisy pressure support [82] or by cyclic large breaths (i.e., using “sighs”) [83] has been shown to safely modulate increased respiratory drive, by improving oxygenation or respiratory mechanics, or through the Hering–Breuer effect, or all the above.
Airway pressure-release ventilation (APRV) is a mode that allows unsupported spontaneous breaths at two pressure levels (low and high) [84]. When APRV is set with a relatively low rate (10–12 bpm) and an inspiratory-to-expiratory (I:E) ratio of 1:1–1:0.8, the non-synchronized mandatory pressure changes generate mechanical breaths that could relieve the patient’s respiratory drive and also be used to estimate the pressure generated by spontaneous breaths (e.g., similar delta pressure for similar Vt) [85].
Pharmacological interventions
Medications that may induce respiratory depression are commonly used for analgo-sedation in ICU patients. However, as most of these medications are associated with short- and long-term adverse effects, their use should be minimized and their effects closely monitored. Use of sedatives or analgesics for the sole purpose of control of respiratory drive may be disadvantageous. It might be more appropriate to look first for the main reason leading to increased respiratory drive (e.g., “fighting the ventilator” or pain) and choose the medication that specifically targets it.
Pain medications
Respiratory depression induced by opioids has long been recognized. A study from 1975 on subcutaneous morphine administered to healthy subjects [86], demonstrated altered ventilatory response to hypercapnia, with decreased slope of the minute ventilation/PaCO2 curve. High doses of intravenous opioids decrease the electrical activity of the inspiratory muscles in opioid-tolerant subject [87]. Opioids are widely used in the ICU for analgo-sedation but only few studies have assessed their effects on respiratory drive. Remifentanyl decreases respiratory rate and increases expiratory timer without modifying EAdi in critically ill patients on assisted ventilation [88]. Reasons for this limited effect might be the use of lower doses compared with those used by opioid abusers and/or the increased respiratory drive of critically ill patients. Thus, opioids might be of limited value for controlling respiratory drive and the risk of P-SILI in ARDS patients.
Drugs modulating agitation and anxiety
Both intravenous and inhaled general anaesthetics reduce the respiratory drive and have been tested in intubated ICU patients, with Propofol showing a more pronounced respiratory depressive effect than isoflurane or sevoflurane [89]. However, the level of sedation needed to obtain a significant impact of such mediations on the respiratory drive might be too deep to be clinically acceptable.
Dexmedetomidine has recently emerged as an alternative drug for awake sedation with the potential of reducing the incidence of delirium. However, dexmedetomidine does not affect the hypercapnic ventilatory response in healthy volunteers [90] and does not modify respiratory rate and gas exchanges in ICU patients compared to placebo [91].
Benzodiazepines are associated with many adverse effects in ICU patients and may be inferior to other sedatives, as suggested by multiple clinical trials [92]. Using benzodiazepines to suppress respiratory drive might not be an optimal approach in most patients.
New pharmacological perspectives
Finally, a recent study suggested that partial muscular paralysis by low-dose neuromuscular blocking agents could obtain protective tidal volumes and inspiratory pressures in patients with acute respiratory failure and uncontrolled high respiratory drive during assisted ventilation [52]. However, it is important to note that the use of neuromuscular blocking agents will induce a sudden uncoupling between respiratory drive and muscular efficiency and its impact on the respiratory drive and patient comfort needs to be better assessed and understood.
Non-pharmacological interventions
Future development of control of respiratory drive in hypoxemic patients might be related to non-pharmacological interventions such as targeted music therapy and extracorporeal CO2 removal (ECCO2R). Previous studies have described possible feed forward interaction between music rhythm and the breathing pattern of healthy and ICU subjects: this generates the fascinating hypothesis that music could act as a modulator of respiratory drive [93], potentially able to override metabolic inputs by decreasing stress and anxiety and increasing comfort (i.e., decreasing the behavioural drive) [94].
ECCO2R decreases the amount of CO2 that must be eliminated through the lungs: this, rather than modifying the brain neural drive, will simply move the metabolic hyperbola downward, thus reducing the actual PaCO2 and minute ventilation level. In the case of stable subjects recovering from ARDS, in whom the slope of brain drive is less steep and the metabolic hyperbola closer to healthy subjects, the decrease of VCO2 through the natural lung by ECCO2R decreases ventilation to minimal levels [95]. In the most severe patients with extremely high respiratory drive and with the metabolic hyperbola significantly shifted upward, efficacy of ventilation reduction by ECCO2R should be more limited, as indicated by pilot data [96]. Moreover, to date, the burden of ECCO2R-related complications is too high to consider the control of respiratory drive an indication for its use, in non-intubated patients with less severe ARDS. As ECCO2R systems become safer with advances in the technology, and the risk-to-benefit ratio improves, ECCO2R might become a more attractive method of controlling respiratory drive and avoiding further lung injury in patients with ARDS.
Conclusions
Respiratory drive may represent a unique synthesis of complex pathophysiologic mechanisms underlying and accompanying ARDS. Higher drive not only may correlate with ARDS severity but, if not carefully managed, could contribute to lung and diaphragm injury. Thus, monitoring of the respiratory drive and interventions able to confine its effects within physiologic limits should be top priorities for the ICU physician caring for subjects with ARDS.
References
Rekling JC, Feldman JL (1998) PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol 60:385–405
Del Negro C, Hayes J (2008) A ‘group pacemaker’ mechanism for respiratory rhythm. generation. J Physiol 586:2245–2246
Smith CA, Rodman JR, Chenuel BJ, Henderson KS, Dempsey JA (2006) Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. J Appl Physiol (1985) 100:13–19
Mohan R, Duffin J (1997) The effect of hypoxia on the ventilatory response to carbon dioxide in man. Respir Physiol 108:101–115
Forster HV, Pan LG, Lowry TF, Serra A, Wenninger J, Martino P (2000) Important role of carotid chemoreceptor afferents in control of breathing of adult and neonatal mammals. Respir Physiol 119:199–208
Eldridge FL (1994) Central integration of mechanisms in exercise hyperpnea. Med Sci Sports Exerc 26:319–327
Tobin MJ, Perez W, Guenther SM, D'Alonzo G, Dantzker DR (1985) (1986) Breathing pattern and metabolic behavior during anticipation of exercise. J Appl Physiol 60:1306–1312
Mahamed S, Ali AF, Ho D, Wang B, Duffin J (2001) The contribution of chemoreflex drives to resting breathing in man. Exp Physiol 86:109–116
Fink B, Hanks E, Ngai S, Papper E (1963) Central regulation of respiration during anesthesia and wakefulness. Ann N Y Acad Sci 109:892–900
Evans KC, Dougherty DD, Schmid AM, Scannell E, McCallister A, Benson H, Dusek JA, Lazar SW (2009) Modulation of spontaneous breathing via limbic/paralimbic-bulbar circuitry: an event-related fMRI study. Neuroimage 47:961–971
Vaporidi K, Akoumianaki E, Telias I, Goligher EC, Brochard L, Georgopoulos D, (2019) Respiratory drive in critically ill patients: pathophysiology and clinical implications. Am J Respir Crit Care Med
Ferluga M, Lucangelo U, Blanch L (2018) Dead space in acute respiratory distress syndrome. Ann Transl Med 6:388
Gattinoni L, Pesenti A (2005) The concept of "baby lung". Intensive Care Med 31:776–784
Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG (2004) Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7:1360–1369
Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG (2006) Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol 572:503–523
Linton RA, Poole-Wilson PA, Davies RJ, Cameron IR (1973) A comparison of the ventilatory response to carbon dioxide by steady-state and rebreathing methods during metabolic acidosis and alkalosis. Clin Sci Mol Med 45:239–249
Jacono FJ, Peng YJ, Nethery D, Faress JA, Lee Z, Kern JA, Prabhakar NR (1985) (2006) Acute lung injury augments hypoxic ventilatory response in the absence of systemic hypoxemia. J Appl Physiol 101:1795–1802
Bauer TT, Montón C, Torres A, Cabello H, Fillela X, Maldonado A, Nicolás JM, Zavala E (2000) Comparison of systemic cytokine levels in patients with acute respiratory distress syndrome, severe pneumonia, and controls. Thorax 55:46–52
Paintal AS (1973) Vagal sensory receptors and their reflex effects. Physiol Rev 53:159–227
Lin S, Walker J, Xu L, Gozal D, Yu J (2007) Behaviours of pulmonary sensory receptors during development of acute lung injury in the rabbit. Exp Physiol 92:749–755
Lee LY, Pisarri TE (2001) Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol 125:47–65
Trenchard D, Gardner D, Guz A (1972) Role of pulmonary vagal afferent nerve fibres in the development of rapid shallow breathing in lung inflammation. Clin Sci 42:251–263
Pisarri TE, Yu J, Coleridge HM, Coleridge JC (1986) Background activity in pulmonary vagal C-fibers and its effects on breathing. Respir Physiol 64:29–43
Jacono FJ, Mayer CA, Hsieh YH, Wilson CG, Dick TE (2011) Lung and brainstem cytokine levels are associated with breathing pattern changes in a rodent model of acute lung injury. Respir Physiol Neurobiol 178:429–438
Hamilton RD, Winning AJ, Horner RL, Guz A (1988) The effect of lung inflation on breathing in man during wakefulness and sleep. Respir Physiol 73:145–154
Moreira TS, Takakura AC, Colombari E, West GH, Guyenet PG (2007) Inhibitory input from slowly adapting lung stretch receptors to retrotrapezoid nucleus chemoreceptors. J Physiol 580:285–300
Morais CCA, Koyama Y, Yoshida T, Plens GM, Gomes S, Lima CAS, Ramos OPS, Pereira SM, Kawaguchi N, Yamamoto H, Uchiyama A, Borges JB, Vidal Melo MF, Tucci MR, Amato MBP, Kavanagh BP, Costa ELV, Fujino Y (2018) High positive end-expiratory pressure renders spontaneous effort noninjurious. Am J Respir Crit Care Med 197:1285–1296
Tipton MJ, Harper A, Paton JFR, Costello JT (2017) The human ventilatory response to stress: rate or depth? J Physiol 595:5729–5752
Rozé H, Germain A, Perrier V, Dewitte A, Joannes-Boyau O, Fleureau C, Ouattara A (2015) Effect of flumazenil on diaphragm electrical activation during weaning from mechanical ventilation after acute respiratory distress syndrome. Br J Anaesth 114:269–275
Pham T, Telias I, Piraino T, Yoshida T, Brochard LJ (2018) Asynchrony consequences and management. Crit Care Clin 34:325–341
Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y (2013) The comparison of spontaneous breathing and muscle paralysis in two different severities of experimental lung injury. Crit Care Med 41:536–545
Luo YM, Moxham J, Polkey MI (2008) Diaphragm electromyography using an oesophageal catheter: current concepts. Clin Sci (Lond) 115:233–244
Piquilloud L, Beloncle F, Richard JM, Mancebo J, Mercat A, Brochard L (2019) Information conveyed by electrical diaphragmatic activity during unstressed, stressed and assisted spontaneous breathing: a physiological study. Ann Intensive Care 9:89
Rozé H, Lafrikh A, Perrier V, Germain A, Dewitte A, Gomez F, Janvier G, Ouattara A (2011) Daily titration of neurally adjusted ventilatory assist using the diaphragm electrical activity. Intensive Care Med 37:1087–1094
Colombo D, Cammarota G, Bergamaschi V, De Lucia M, Corte FD, Navalesi P (2008) Physiologic response to varying levels of pressure support and neurally adjusted ventilatory assist in patients with acute respiratory failure. Intensive Care Med 34:2010–2018
Liu L, Liu H, Yang Y, Huang Y, Liu S, Beck J, Slutsky AS, Sinderby C, Qiu H (2012) Neuroventilatory efficiency and extubation readiness in critically ill patients. Crit Care 16:R143
Parthasarathy S, Jubran A, Laghi F, Tobin MJ (1985) (2007) Sternomastoid, rib cage, and expiratory muscle activity during weaning failure. J Appl Physiol 103:140–147
Shi ZH, Jonkman A, de Vries H, Jansen D, Ottenheijm C, Girbes A, Spoelstra-de Man A, Zhou JX, Brochard L, Heunks L (2019) Expiratory muscle dysfunction in critically ill patients: towards improved understanding. Intensive Care Med 45:1061–1071
Pellegrini M, Hedenstierna G, Roneus A, Segelsjö M, Larsson A, Perchiazzi G (2017) The diaphragm acts as a brake during expiration to prevent lung collapse. Am J Respir Crit Care Med 195:1608–1616
Schmidt M, Kindler F, Gottfried SB, Raux M, Hug F, Similowski T, Demoule A (2013) Dyspnea and surface inspiratory electromyograms in mechanically ventilated patients. Intensive Care Med 39:1368–1376
Mauri T, Yoshida T, Bellani G, Goligher EC, Carteaux G, Rittayamai N, Mojoli F, Chiumello D, Piquilloud L, Grasso S, Jubran A, Laghi F, Magder S, Pesenti A, Loring S, Gattinoni L, Talmor D, Blanch L, Amato M, Chen L, Brochard L, Mancebo J (2016) Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med 42:1360–1373
Bertoni M, Telias I, Urner M, Long M, Del Sorbo L, Fan E, Sinderby C, Beck J, Liu L, Qiu H, Wong J, Slutsky AS, Ferguson ND, Brochard LJ, Goligher EC (2019) A novel non-invasive method to detect excessively high respiratory effort and dynamic transpulmonary driving pressure during mechanical ventilation. Crit Care 23:346
Carteaux G, Mancebo J, Mercat A, Dellamonica J, Richard JC, Aguirre-Bermeo H, Kouatchet A, Beduneau G, Thille AW, Brochard L (2013) Bedside adjustment of proportional assist ventilation to target a predefined range of respiratory effort. Crit Care Med 41:2125–2132
Whitelaw WA, Derenne JP, Milic-Emili J (1975) Occlusion pressure as a measure of respiratory center output in conscious man. Respir Physiol 23:181–199
Rittayamai N, Beloncle F, Goligher EC, Chen L, Mancebo J, Richard JM, Brochard L (2017) Effect of inspiratory synchronization during pressure-controlled ventilation on lung distension and inspiratory effort. Ann Intensive Care 7:100
Alberti A, Gallo F, Fongaro A, Valenti S, Rossi A (1995) P0.1 is a useful parameter in setting the level of pressure support ventilation. Intensive Care Med 21:547–553
Holle RH, Schoene RB, Pavlin EJ (1984) Effect of respiratory muscle weakness on P0.1 induced by partial curarization. J Appl Physiol Respir Environ Exerc Physiol 57:1150–1157
Clark FJ, von Euler C (1972) On the regulation of depth and rate of breathing. J Physiol 222:267–295
Hey EN, Lloyd BB, Cunningham DJ, Jukes MG, Bolton DP (1966) Effects of various respiratory stimuli on the depth and frequency of breathing in man. Respir Physiol 1:193–205
Georgopoulos D, Mitrouska I, Bshouty Z, Webster K, Patakas D, Younes M (1997) Respiratory response to CO2 during pressure-support ventilation in conscious normal humans. Am J Respir Crit Care Med 156:146–154
Carteaux G, Millán-Guilarte T, De Prost N, Razazi K, Abid S, Thille AW, Schortgen F, Brochard L, Brun-Buisson C, Mekontso Dessap A (2016) Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: role of tidal volume. Crit Care Med 44:282–290
Doorduin J, Nollet JL, Roesthuis LH, van Hees HW, Brochard LJ, Sinderby CA, van der Hoeven JG, Heunks LM (2017) Partial neuromuscular blockade during partial ventilatory support in sedated patients with high tidal volumes. Am J Respir Crit Care Med 195:1033–1042
Arnal JM, Wysocki M, Nafati C, Donati S, Granier I, Corno G, Durand-Gasselin J (2008) Automatic selection of breathing pattern using adaptive support ventilation. Intensive Care Med 34:75–81
Kallet RH, Hemphill JC, Dicker RA, Alonso JA, Campbell AR, Mackersie RC, Katz JA (2007) The spontaneous breathing pattern and work of breathing of patients with acute respiratory distress syndrome and acute lung injury. Respir Care 52:989–995
Tobin MJ, Perez W, Guenther SM, Semmes BJ, Mador MJ, Allen SJ, Lodato RF, Dantzker DR (1986) The pattern of breathing during successful and unsuccessful trials of weaning from mechanical ventilation. Am Rev Respir Dis 134:1111–1118
Yang KL, Tobin MJ (1991) A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med 324:1445–1450
Brack T, Jubran A, Tobin MJ (1997) Effect of elastic loading on variational activity of breathing. Am J Respir Crit Care Med 155:1341–1348
Brochard L, Slutsky A, Pesenti A (2017) Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med 195:438–442
Schmidt M, Banzett RB, Raux M, Morélot-Panzini C, Dangers L, Similowski T, Demoule A (2014) Unrecognized suffering in the ICU: addressing dyspnea in mechanically ventilated patients. Intensive Care Med 40:1–10
McCloskey D (1981) Corollary discharges: motor commands and perception. In: Brooks, VB (eds) Handbook of physiology. American Physiological Society, Washington, D.C.
Widdicombe J (2009) Lung afferent activity: implications for respiratory sensation. Respir Physiol Neurobiol 167:2–8
Peiffer C, Poline JB, Thivard L, Aubier M, Samson Y (2001) Neural substrates for the perception of acutely induced dyspnea. Am J Respir Crit Care Med 163:951–957
Leray V, Bourdin G, Flandreau G, Bayle F, Wallet F, Richard JC, Guérin C (2010) A case of pneumomediastinum in a patient with acute respiratory distress syndrome on pressure support ventilation. Respir Care 55:770–773
Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y (2012) Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Crit Care Med 40:1578–1585
Yoshida T, Nakahashi S, Nakamura MAM, Koyama Y, Roldan R, Torsani V, De Santis RR, Gomes S, Uchiyama A, Amato MBP, Kavanagh BP, Fujino Y (2017) Volume-controlled ventilation does not prevent injurious inflation during spontaneous effort. Am J Respir Crit Care Med 196:590–601
Yoshida T, Torsani V, Gomes S, De Santis RR, Beraldo MA, Costa EL, Tucci MR, Zin WA, Kavanagh BP, Amato MB (2013) Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med 188:1420–1427
Yoshida T, Amato MBP, Kavanagh BP, Fujino Y (2019) Impact of spontaneous breathing during mechanical ventilation in acute respiratory distress syndrome. Curr Opin Crit Care 25:192–198
Vassilakopoulos T, Divangahi M, Rallis G, Kishta O, Petrof B, Comtois A, Hussain SN (2004) Differential cytokine gene expression in the diaphragm in response to strenuous resistive breathing. Am J Respir Crit Care Med 170:154–161
Wang X, Jiang TX, Road JD, Redenbach DM, Reid WD (2005) Granulocytosis and increased adhesion molecules after resistive loading of the diaphragm. Eur Respir J 26:786–794
Reid WD, Belcastro AN (2000) Time course of diaphragm injury and calpain activity during resistive loading. Am J Respir Crit Care Med 162:1801–1806
Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, Rittayamai N, Lanys A, Tomlinson G, Singh JM, Bolz SS, Rubenfeld GD, Kavanagh BP, Brochard LJ, Ferguson ND (2015) Evolution of diaphragm thickness during mechanical ventilation. impact of inspiratory effort. Am J Respir Crit Care Med 192:1080–1088
Mauri T, Wang YM, Dalla Corte F, Corcione N, Spinelli E, Pesenti A (2019) Nasal high flow: physiology, efficacy and safety in the acute care setting, a narrative review. Open Access Emerg Med 11:109–120
Patroniti N, Foti G, Manfio A, Coppo A, Bellani G, Pesenti A (2003) Head helmet versus face mask for non-invasive continuous positive airway pressure: a physiological study. Intensive Care Med 29:1680–1687
Mauri T, Alban L, Turrini C, Cambiaghi B, Carlesso E, Taccone P, Bottino N, Lissoni A, Spadaro S, Volta CA, Gattinoni L, Pesenti A, Grasselli G (2017) Optimum support by high-flow nasal cannula in acute hypoxemic respiratory failure: effects of increasing flow rates. Intensive Care Med 43:1453–1463
Brambilla AM, Aliberti S, Prina E, Nicoli F, Del Forno M, Nava S, Ferrari G, Corradi F, Pelosi P, Bignamini A, Tarsia P, Cosentini R (2014) Helmet CPAP vs. oxygen therapy in severe hypoxemic respiratory failure due to pneumonia. Intensive Care Med 40:942–949
L'Her E, Deye N, Lellouche F, Taille S, Demoule A, Fraticelli A, Mancebo J, Brochard L (2005) Physiologic effects of noninvasive ventilation during acute lung injury. Am J Respir Crit Care Med 172:1112–1118
Grieco DL, Menga LS, Raggi V, Bongiovanni F, Anzellotti GM, Tanzarella ES, Bocci MG, Mercurio G, Dell'Anna AM, Eleuteri D, Bello G, Maviglia R, Conti G, Maggiore SM, Antonelli M, (2019) Physiological comparison of high-flow nasal cannula and helmet noninvasive ventilation in acute hypoxemic respiratory failure. Am J Respir Crit Care Med
Pesenti A, Rossi N, Calori A, Foti G, Rossi GP (1993) Effects of short-term oxygenation changes on acute lung injury patients undergoing pressure support ventilation. Chest 103:1185–1189
Volta CA, Alvisi V, Bertacchini S, Marangoni E, Ragazzi R, Verri M, Alvisi R (2006) Acute effects of hyperoxemia on dyspnoea and respiratory variables during pressure support ventilation. Intensive Care Med 32:223–229
Patroniti N, Bellani G, Saccavino E, Zanella A, Grasselli G, Isgrò S, Milan M, Foti G, Pesenti A (2012) Respiratory pattern during neurally adjusted ventilatory assist in acute respiratory failure patients. Intensive Care Med 38:230–239
Vaporidi K, Psarologakis C, Proklou A, Pediaditis E, Akoumianaki E, Koutsiana E, Chytas A, Chouvarda I, Kondili E, Georgopoulos D (2019) Driving pressure during proportional assist ventilation: an observational study. Ann Intensive Care 9:1
Spieth PM, Carvalho AR, Güldner A, Kasper M, Schubert R, Carvalho NC, Beda A, Dassow C, Uhlig S, Koch T, Pelosi P, Gama de Abreu M (2011) Pressure support improves oxygenation and lung protection compared to pressure-controlled ventilation and is further improved by random variation of pressure support. Crit Care Med 39:746–755
Mauri T, Eronia N, Abbruzzese C, Marcolin R, Coppadoro A, Spadaro S, Patroniti N, Bellani G, Pesenti A (2015) Effects of sigh on regional lung strain and ventilation heterogeneity in acute respiratory failure patients undergoing assisted mechanical ventilation. Crit Care Med 43:1823–1831
Rose L, Hawkins M (2008) Airway pressure release ventilation and biphasic positive airway pressure: a systematic review of definitional criteria. Intensive Care Med 34:1766–1773
Rozé H, Richard JM, Thumerel M, Ouattara A (2017) Spontaneous breathing (SB) using airway pressure-release ventilation (APRV) in patients under extracorporeal-membrane oxygenation (ECMO) for acute respiratory distress syndrome (ARDS). Intensive Care Med 43:1919–1920
Weil JV, McCullough RE, Kline JS, Sodal IE (1975) Diminished ventilatory response to hypoxia and hypercapnia after morphine in normal man. N Engl J Med 292:1103–1106
Jolley CJ, Bell J, Rafferty GF, Moxham J, Strang J (2015) Understanding heroin overdose: a study of the acute respiratory depressant effects of injected pharmaceutical heroin. PLoS ONE 10:e0140995
Costa R, Navalesi P, Cammarota G, Longhini F, Spinazzola G, Cipriani F, Ferrone G, Festa O, Antonelli M, Conti G (2017) Remifentanil effects on respiratory drive and timing during pressure support ventilation and neurally adjusted ventilatory assist. Respir Physiol Neurobiol 244:10–16
Migliari M, Bellani G, Rona R, Isgrò S, Vergnano B, Mauri T, Patroniti N, Pesenti A, Foti G (2009) Short-term evaluation of sedation with sevoflurane administered by the anesthetic conserving device in critically ill patients. Intensive Care Med 35:1240–1246
Hsu YW, Cortinez LI, Robertson KM, Keifer JC, Sum-Ping ST, Moretti EW, Young CC, Wright DR, Macleod DB, Somma J (2004) Dexmedetomidine pharmacodynamics: part I: crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology 101:1066–1076
Venn RM, Hell J, Grounds RM (2000) Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care 4:302–308
Ely EW, Dittus RS, Girard TD (2012) Point: should benzodiazepines be avoided in mechanically ventilated patients? Yes. Chest 142:281–284
Haas F, Distenfeld S, Axen K (1985) (1986) Effects of perceived musical rhythm on respiratory pattern. J Appl Physiol 61:1185–1191
Chlan LL, Weinert CR, Heiderscheit A, Tracy MF, Skaar DJ, Guttormson JL, Savik K (2013) Effects of patient-directed music intervention on anxiety and sedative exposure in critically ill patients receiving mechanical ventilatory support: a randomized clinical trial. JAMA 309:2335–2344
Mauri T, Grasselli G, Suriano G, Eronia N, Spadaro S, Turrini C, Patroniti N, Bellani G, Pesenti A (2016) Control of respiratory drive and effort in extracorporeal membrane oxygenation patients recovering from severe acute respiratory distress syndrome. Anesthesiology 125:159–167
Crotti S, Bottino N, Ruggeri GM, Spinelli E, Tubiolo D, Lissoni A, Protti A, Gattinoni L (2017) Spontaneous breathing during extracorporeal membrane oxygenation in acute respiratory failure. Anesthesiology 126:678–687
Acknowledgements
The present review was supported, in part by Departmental funding (ES and TM). The funding source didn’t have any role in any aspect of the present work. The corresponding author had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
ES, TM and DB conceived the review. ES and TM conducted the articles search and drafted the manuscript. All authors revised the manuscript for critical intellectual content.
Corresponding author
Ethics declarations
Conflicts of interest
ES and JRB do not have any conflict of interests to disclose. TM reports personal fees from Drager, Fisher and Paykel and Mindray, outside the submitted work. AP reports personal fees from Maquet, Novalung/Xenios, Baxter and Boehringer Ingelheim, outside the submitted work. DB reports grants from ALung technologies, personal fees from Baxter, personal fees from BREETHE, personal fees from Xenios, other from Hemovent, outside the submitted work.
Search strategy and selection criteria
References for this review were identified through searches of PubMed for articles published from January 1950 to July 2019, by use of the terms “respiratory drive”, “spontaneous breathing”, and “acute respiratory distress syndrome”. Articles resulting from these searches and relevant references cited in those articles were reviewed. Articles published in English were included.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Spinelli, E., Mauri, T., Beitler, J.R. et al. Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med 46, 606–618 (2020). https://doi.org/10.1007/s00134-020-05942-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-020-05942-6