Abstract
Purpose
Idiopathic pulmonary fibrosis (IPF) is the most common idiopathic interstitial pneumonia and its prognosis is poor. Epidemiological evidence suggests an association of IPF with vascular disease and thrombotic tendency, which may be related to platelet activation.
Methods
Platelet–monocyte adhesion in peripheral blood was examined by flow cytometry in patients with IPF (n = 19), interstitial lung disease (ILD) other than IPF (n = 9), and control subjects without pulmonary fibrosis (n = 14). Expression of platelet activation markers P-selectin (CD62P), PSGL-1 (CD162), and CD40 ligand (CD40L) on leukocytes and platelets were studied. Plasma concentrations of soluble P-selectin and CD40L were measured by ELISA.
Results
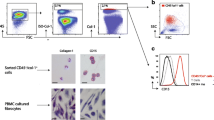
Significantly elevated levels of platelet–monocyte binding were found in patients with IPF (35.6 ± 4.34 % [mean ± SEM]) compared with patients with non-IPF ILD (23.5 ± 3.68 %) and non-ILD control subjects (16.5 ± 2.26 %; P < 0.01). There was a trend towards increased divalent cation-independent platelet–monocyte binding in IPF (6.0 ± 0.77 % [mean ± SEM]) compared with non-IPF ILD (4.3 ± 1.38 %) and control subjects without ILD (3.1 ± 1.75 %; P = 0.058). There was no differential surface expression of platelet activation markers on subsets of leukocytes or platelets. Plasma concentrations of CD40L and soluble P-selectin did not differ between IPF and control subjects. Platelet–monocyte binding had no significant correlation with percent predicted TLco or FVC.
Conclusions
Platelet–monocyte binding is increased in IPF, suggesting increased platelet activation. This conjugation is predominantly calcium-dependent, but there may be more calcium-independent adhesion in IPF. These findings support further research into the role of platelet activation in IPF.






Similar content being viewed by others
Abbreviations
- CD40L:
-
CD40 ligand
- FACS:
-
Fluorescent activated cell sorting
- IPF:
-
Idiopathic pulmonary fibrosis
- ILD:
-
Interstitial lung disease
- mAb:
-
Monoclonal antibodies
- PMN:
-
Polymorphonuclear leukocytes
References
American Thoracic Society, European Respiratory Society (2002) American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 165:277–304
Kim DS, Collard HR, King TE Jr (2006) Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc 3:285–292
Hubbard RB, Smith C, Le JI, Gribbin J, Fogarty AW (2008) The association between idiopathic pulmonary fibrosis and vascular disease: a population-based study. Am J Respir Crit Care Med 178:1257–1261
Daniels CE, Yi ES, Ryu JH (2008) Autopsy findings in 42 consecutive patients with idiopathic pulmonary fibrosis. Eur Respir J 32:170–174
Parra ER, David YR, da Costa LR et al (2005) Heterogeneous remodeling of lung vessels in idiopathic pulmonary fibrosis. Lung 183:291–300
Ebina M, Shimizukawa M, Shibata N et al (2004) Heterogeneous increase in CD34-positive alveolar capillaries in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 169:1203–1208
Colombat M, Mal H, Groussard O et al (2007) Pulmonary vascular lesions in end-stage idiopathic pulmonary fibrosis: histopathologic study on lung explant specimens and correlations with pulmonary hemodynamics. Hum Pathol 38:60–65
Dees C, Akhmetshina A, Zerr P et al (2011) Platelet-derived serotonin links vascular disease and tissue fibrosis. J Exp Med 208(5):961–972
Antoniades HN, Bravo MA, Avila RE et al (1990) Platelet-derived growth factor in idiopathic pulmonary fibrosis. J Clin Invest 86:1055–1064
Bitterman PB, Adelberg S, Crystal RG (1983) Mechanisms of pulmonary fibrosis. Spontaneous release of the alveolar macrophage-derived growth factor in the interstitial lung disorders. J Clin Invest 72:1801–1813
Bitterman PB, Rennard SI, Adelberg S, Crystal RG (1983) Role of fibronectin as a growth factor for fibroblasts. J Cell Biol 97:1925–1932
Bonner JC (2004) Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev 15:255–273
Scotton CJ, Chambers RC (2007) Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest 132:1311–1321
Davi G, Patrono C (2007) Platelet activation and atherothrombosis. N Engl J Med 357:2482–2494
Neumann FJ, Marx N, Gawaz M et al (1997) Induction of cytokine expression in leukocytes by binding of thrombin-stimulated platelets. Circulation 95:2387–2394
Theilmeier G, Lenaerts T, Remacle C, Collen D, Vermylen J, Hoylaerts MF (1999) Circulating activated platelets assist THP-1 monocytoid/endothelial cell interaction under shear stress. Blood 94:2725–2734
Merhi Y, Provost P, Guidoin R, Latour JG (1997) Importance of platelets in neutrophil adhesion and vasoconstriction after deep carotid arterial injury by angioplasty in pigs. Arterioscler Thromb Vasc Biol 17:1185–1191
Sarma J, Laan CA, Alam S, Jha A, Fox KA, Dransfield I (2002) Increased platelet binding to circulating monocytes in acute coronary syndromes. Circulation 105:2166–2171
Maclay JD, McAllister DA, Johnston S et al (2011) Increased platelet activation in patients with stable and acute exacerbation of COPD. Thorax 66:769–774
American Thoracic Society (2000) Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 161:646–664 [Review] [234 refs]
Bournazos S, Rennie J, Hart SP, Dransfield I (2008) Choice of anticoagulant critically affects measurement of circulating platelet–leukocyte complexes. Arterioscler Thromb Vasc Biol 28:e2–e3
Frenette PS, Denis CV, Weiss L et al (2000) P-selectin glycoprotein ligand 1 (PSGL-1) is expressed on platelets and can mediate platelet–endothelial interactions in vivo. J Exp Med 191:1413–1422
Armitage RJ, Fanslow WC, Strockbine L et al (1992) Molecular and biological characterization of a murine ligand for CD40. Nature 357:80–82
Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A (1992) A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci USA 89:6550–6554
Grammer AC, Bergman MC, Miura Y, Fujita K, Davis LS, Lipsky PE (1995) The CD40 ligand expressed by human B cells costimulates B cell responses. J Immunol 154:4996–5010
Pinchuk LM, Klaus SJ, Magaletti DM, Pinchuk GV, Norsen JP, Clark EA (1996) Functional CD40 ligand expressed by human blood dendritic cells is up-regulated by CD40 ligation. J Immunol 157:4363–4370
Henn V, Slupsky JR, Grafe M et al (1998) CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 391:591–594
Kaufman J, Sime PJ, Phipps RP (2004) Expression of CD154 (CD40 ligand) by human lung fibroblasts: differential regulation by IFN-gamma and IL-13, and implications for fibrosis. J Immunol 172:1862–1871
Juul K, Tybjaerg-Hansen A, Mortensen J, Lange P, Vestbo J, Nordestgaard BG (2005) Factor V leiden homozygosity, dyspnea, and reduced pulmonary function. Arch Intern Med 165:2032–2036
Chambers RC, Laurent GJ (2002) Coagulation cascade proteases and tissue fibrosis. Biochem Soc Trans 30:194–200
Howell DC, Laurent GJ, Chambers RC (2002) Role of thrombin and its major cellular receptor, protease-activated receptor-1, in pulmonary fibrosis. Biochem Soc Trans 30:211–216
Howell DC, Johns RH, Lasky JA et al (2005) Absence of proteinase-activated receptor-1 signaling affords protection from bleomycin-induced lung inflammation and fibrosis. Am J Pathol 166:1353–1365
Magro CM, Allen J, Pope-Harman A et al (2003) The role of microvascular injury in the evolution of idiopathic pulmonary fibrosis. Am J Clin Pathol 119:556–567
Bargagli E, Madioni C, Bianchi N, Refini RM, Cappelli R, Rottoli P (2013) Serum analysis of coagulation factors in IPF and NSIP. Inflammation. doi:10.1007/s10753-013-9706-z
Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI (2001) Circulating monocyte–platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation 104:1533–1537
Cicha I, Garlichs CD, Daniel WG, Goppelt-Struebe M (2004) Activated human platelets release connective tissue growth factor. Thromb Haemost 91:755–760
McKeown S, Richter AG, O’Kane C, McAuley DF, Thickett DR (2009) MMP expression and abnormal lung permeability are important determinants of outcome in IPF. Eur Respir J 33:77–84
Katsuda S, Kaji T (2003) Atherosclerosis and extracellular matrix. J Atheroscler Thromb 10:267–274
Schwartz SM, Virmani R, Rosenfeld ME (2000) The good smooth muscle cells in atherosclerosis. Curr Atheroscler Rep 2:422–429
Consigny PM (1995) Pathogenesis of atherosclerosis. AJR Am J Roentgenol 164:553–558
da Costa Martins P, van den Berk N, Ulfman LH, Koenderman L, Hordijk PL, Zwaginga JJ (2004) Platelet–monocyte complexes support monocyte adhesion to endothelium by enhancing secondary tethering and cluster formation. Arterioscler Thromb Vasc Biol 24:193–199
Acknowledgments
The authors thank Dr. L. Sadofsky, Mr. C. Crow, Dr. V. Green, Dr. L. Madden, and Mr. W. Sheedy for their assistance in conducting the experiments during this study. We also acknowledge Dr. V. Allgar for her help with the statistical analyses. The study was funded by the University of Hull.
Conflict of interest
None declared for any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fahim, A., Crooks, M.G., Morice, A.H. et al. Increased Platelet Binding to Circulating Monocytes in Idiopathic Pulmonary Fibrosis. Lung 192, 277–284 (2014). https://doi.org/10.1007/s00408-013-9546-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-013-9546-5