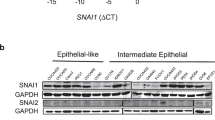
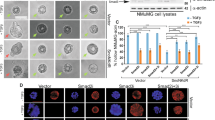
Abstract
Epithelial–mesenchymal transition (EMT) is essential for organogenesis and is triggered during carcinoma progression to an invasive state1. Transforming growth factor-β (TGF-β) cooperates with signalling pathways, such as Ras and Wnt, to induce EMT2,3,4,5, but the molecular mechanisms are not clear. Here, we report that SMAD3 and SMAD4 interact and form a complex with SNAIL1, a transcriptional repressor and promoter of EMT6,7. The SNAIL1–SMAD3/4 complex was targeted to the gene promoters of CAR, a tight-junction protein, and E-cadherin during TGF-β-driven EMT in breast epithelial cells. SNAIL1 and SMAD3/4 acted as co-repressors of CAR, occludin, claudin-3 and E-cadherin promoters in transfected cells. Conversely, co-silencing of SNAIL1 and SMAD4 by siRNA inhibited repression of CAR and occludin during EMT. Moreover, loss of CAR and E-cadherin correlated with nuclear co-expression of SNAIL1 and SMAD3/4 in a mouse model of breast carcinoma and at the invasive fronts of human breast cancer. We propose that activation of a SNAIL1–SMAD3/4 transcriptional complex represents a mechanism of gene repression during EMT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Thiery, J. P. & Sleeman, J. P. Complex networks orchestrate epithelial–mesenchymal transitions. Nature Rev. Mol. Cell Biol. 7, 131–142 (2006).
Janda, E. et al. Ras and TGF-β cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J. Cell Biol. 156, 299–313 (2002).
Massague, J. TGF-β in cancer. Cell 134, 215–230 (2008).
Nawshad, A., Lagamba, D., Polad, A. & Hay, E. D. Transforming growth factor-β signaling during epithelial-mesenchymal transformation: implications for embryogenesis and tumor metastasis. Cells Tissues Organs 179, 11–23 (2005).
Pardali, K. & Moustakas, A. Actions of TGF-β as tumor suppressor and pro-metastatic factor in human cancer. Biochim. Biophys. Acta 1775, 21–62 (2007).
De Craene, B., van Roy, F. & Berx, G. Unraveling signalling cascades for the Snail family of transcription factors. Cell Signal 17, 535–547 (2005).
Yook, J. I. et al. A Wnt-Axin2-GSK3β cascade regulates Snail1 activity in breast cancer cells. Nature Cell Biol. 8, 1398–1406 (2006).
Massague, J. How cells read TGF-β signals. Nature Rev. Mol. Cell Biol. 1, 169–178 (2000).
Bardeesy, N. et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 20, 3130–3146 (2006).
Nieto, M. A. The snail superfamily of zinc-finger transcription factors. Nature Rev. Mol. Cell Biol. 3, 155–166 (2002).
Batlle, E. et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nature Cell Biol. 2, 84–89 (2000).
Cano, A. et al. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nature Cell Biol. 2, 76–83 (2000).
Ikenouchi, J., Matsuda, M., Furuse, M. & Tsukita, S. Regulation of tight junctions during the epithelium–mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J. Cell Sci. 116, 1959–1967 (2003).
Bachelder, R. E., Yoon, S. O., Franci, C., de Herreros, A. G. & Mercurio, A. M. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial–mesenchymal transition. J. Cell Biol. 168, 29–33 (2005).
Zhou, B. P. et al. Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial–mesenchymal transition. Nature Cell Biol. 6, 931–940 (2004).
Franci, C. et al. Expression of Snail protein in tumor–stroma interface. Oncogene 25, 5134–5144 (2006).
Coyne, C. B. & Bergelson, J. M. CAR: a virus receptor within the tight junction. Adv. Drug Deliv. Rev. 57, 869–882 (2005).
Lacher, M. D. et al. Transforming growth factor-β receptor inhibition enhances adenoviral infectability of carcinoma cells via upregulation of Coxsackie and adenovirus receptor in conjunction with reversal of epithelial–mesenchymal transition. Cancer Res. 66, 1648–1657 (2006).
Philipson, L. & Pettersson, R. F. The coxsackie-adenovirus receptor—a new receptor in the immunoglobulin family involved in cell adhesion. Curr. Top. Microbiol. Immunol. 273, 87–111 (2004).
Raschperger, E. et al. The coxsackie- and adenovirus receptor (CAR) is an in vivo marker for epithelial tight junctions, with a potential role in regulating permeability and tissue homeostasis. Exp. Cell Res. 312, 1566–1580 (2006).
Matsumoto, K., Shariat, S. F., Ayala, G. E., Rauen, K. A. & Lerner, S. P. Loss of coxsackie and adenovirus receptor expression is associated with features of aggressive bladder cancer. Urology 66, 441–446 (2005).
Yamashita, M., Ino, A., Kawabata, K., Sakurai, F. & Mizuguchi, H. Expression of coxsackie and adenovirus receptor reduces the lung metastatic potential of murine tumor cells. Int. J. Cancer 121, 1690–1696 (2007).
Anders, M., Christian, C., McMahon, M., McCormick, F. & Korn, W. M. Inhibition of the Raf/MEK/ERK pathway up-regulates expression of the coxsackievirus and adenovirus receptor in cancer cells. Cancer Res. 63, 2088–2095 (2003).
Oft, M. et al. TGF-β1 and Ha–Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 10, 2462–2477 (1996).
Miettinen, P. J., Ebner, R., Lopez, A. R. & Derynck, R. TGF-β induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J. Cell Biol. 127, 2021–2036 (1994).
Mauhin, V., Lutz, Y., Dennefeld, C. & Alberga, A. Definition of the DNA-binding site repertoire for the Drosophila transcription factor SNAIL. Nucleic Acids Res. 21, 3951–3957 (1993).
Nawshad, A., Medici, D., Liu, C. C. & Hay, E. D. TGFβ3 inhibits E-cadherin gene expression in palate medial-edge epithelial cells through a Smad2–Smad4–LEF1 transcription complex. J. Cell Sci. 120, 1646–1653 (2007).
Shirakihara, T., Saitoh, M. & Miyazono, K. Differential regulation of epithelial and mesenchymal markers by δEF1 proteins in epithelial mesenchymal transition induced by TGF-β. Mol. Biol. Cell 18, 3533–3544 (2007).
Mahoney, K. H., Miller, B. E. & Heppner, G. H. FACS quantitation of leucine aminopeptidase and acid phosphatase on tumor-associated macrophages from metastatic and nonmetastatic mouse mammary tumors. J. Leukoc. Biol. 38, 573–585 (1985).
Saitou, M. et al. Mammalian occludin in epithelial cells: its expression and subcellular distribution. Eur. J. Cell Biol. 73, 222–231 (1997).
Takkunen, M. et al. Snail-dependent and -independent epithelial-mesenchymal transition in oral squamous carcinoma cells. J. Histochem. Cytochem. 54, 1263–1275 (2006).
Meijer, L. et al. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem. Biol. 10, 1255–1266 (2003).
Jinnin, M., Ihn, H. & Tamaki, K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-β1-induced extracellular matrix expression. Mol. Pharmacol. 69, 597–607 (2006).
Pardali, K. et al. Role of Smad proteins and transcription factor Sp1 in p21(Waf1/Cip1) regulation by transforming growth factor-β. J. Biol. Chem. 275, 29244–29256 (2000).
Hajra, K. M., Chen, D. Y. & Fearon, E. R. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 62, 1613–1618 (2002).
Frey, B. M. et al. High-efficiency gene transfer into ex vivo expanded human hematopoietic progenitors and precursor cells by adenovirus vectors. Blood 91, 2781–2792 (1998).
Wei, W.Z. et al. Protection against mammary tumor growth by vaccination with full-length, modified human ErbB2 DNA. Int. J. Cancer 81, 748–754 (1999).
Acknowledgements
We thank Carl-Henrik Heldin (Ludwig Institute for Cancer Research, Uppsala Branch) and Ola Hermansson (Department of Neuroscience, Karolinska Institute, Sweden) for fruitful discussions; Clara Francí (IMIM-Hospital del Mar, Barcelona) for the initial immunohistochemical analysis of human tumors; Anita Bergström, Silvia Menéndez and Marta Garrido for excellent technical assistance and Olov Andersson (Department of Cell and Molecular Biology, Karolinska Institute) for antibodies. Jonas Fuxe was supported by grants from the Swedish Research Council, the Swedish Wenner-Gren Foundation, the Swedish Childhood Cancer Foundation and an International Union Against Cancer (UICC), American Cancer Society International Fellowship for Beginning Investigators. Theresa Vincent was supported by the Swedish Research Council. Philip Leopold and Ronald Crystal were supported by the National Institutes of Health (NIH) by PO1 HL59312 and Antonio García de Herreros, Joan Albanell and Federico Rojo by RD06/0020/109, RD06/0020/040, FIS PI061513, SAF2006-00339 and Fundació Privada Cellex.
Author information
Authors and Affiliations
Contributions
T.V., E.P.A.N. and J.F. initiated the research, designed experiments and analysed data. T.V., E.P.A.N. and J.R.J. performed the in vitro experiments and discussed and analysed data. J.F. supervised the work and wrote the manuscript. T.V., E.P.A.N. and J.R.J. provided substantial input into preparation of the manuscript. F.R. and J.A. performed studies involving human tumours and analysed data. A.G.deH. designed and supervised studies on the human tumour samples. A.M. initiated experiments and discussed data involving SMADs. A.K. worked on protein interactions. K.P. performed experiments and analysed data from the mouse model. I.V. provided essential reagents. L.P., R.F.P., P.L.L. and R.G.C. discussed data, initiated experiments and provided significant intellectual contributions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1750 kb)
Rights and permissions
About this article
Cite this article
Vincent, T., Neve, E., Johnson, J. et al. A SNAIL1–SMAD3/4 transcriptional repressor complex promotes TGF-β mediated epithelial–mesenchymal transition. Nat Cell Biol 11, 943–950 (2009). https://doi.org/10.1038/ncb1905
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1905
This article is cited by
-
EMT/MET plasticity in cancer and Go-or-Grow decisions in quiescence: the two sides of the same coin?
Molecular Cancer (2023)
-
GATA zinc finger protein p66β promotes breast cancer cell migration by acting as a co-activator of Snail
Cell Death & Disease (2023)
-
TGFß1 Stimulates Lymphatic Endothelial Cells to Produce IL7 and IL15, Which Act as Chemotactic Factors for Breast Cancer Cells with Mesenchymal Properties
Journal of Mammary Gland Biology and Neoplasia (2023)
-
Long noncoding RNA Smyca coactivates TGF-β/Smad and Myc pathways to drive tumor progression
Journal of Hematology & Oncology (2022)
-
Prognostic value of functional SMAD4 localization in extrahepatic bile duct cancer
World Journal of Surgical Oncology (2022)