Abstract
Neutrophil extracellular traps (NETs) extruded from neutrophils upon activation are composed of chromatin associated with cytosolic and granular proteins, which ensnare and kill microorganisms. This microbicidal mechanism named classical netosis has been shown to dependent on reactive oxygen species (ROS) generation by NADPH oxidase and also chromatin decondensation dependent upon the enzymes (PAD4), neutrophil elastase (NE) and myeloperoxidase (MPO). NET release also occurs through an early/rapid ROS-independent mechanism, named early/rapid vital netosis. Here we analyze the role of ROS, NE, MPO and PAD4 in the netosis stimulated by Leishmania amazonensis promastigotes in human neutrophils. We demonstrate that promastigotes induce a classical netosis, dependent on the cellular redox imbalance, as well as by a chloroamidine sensitive and elastase activity mechanism. Additionally, Leishmania also induces the early/rapid NET release occurring only 10 minutes after neutrophil-parasite interaction. We demonstrate here, that this early/rapid mechanism is dependent on elastase activity, but independent of ROS generation and chloroamidine. A better understanding of both mechanisms of NET release and the NETs effects on the host immune system modulation, could support the development of new potential therapeutic strategies for leishmaniasis.
Similar content being viewed by others
Introduction
Neutrophils are the most abundant leukocytes in blood and play an important role in the innate immune response. They are the first cells to arrive at an infection site and are endowed with potent antimicrobial mechanisms. Netosis is one of these mechanisms and occurs with the release of a scaffold of chromatin associated with different granular and intracellular proteins, named neutrophil extracellular traps (NETs)1,2. NET release can be triggered by several stimuli, among them, pathogens such as Leishmania3,4.
The molecular mechanisms behind NET formation are still poorly understood. To date two main NET release mechanisms have been proposed: the classical, reactive oxygen species (ROS)-dependent and the early/rapid ROS-independent5,6. In the classical mechanism, neutrophils enter a cell death program that culminates with the release of NETs 1–4 hours after activation, in a process that is dependent on ROS production. The majority of the stimuli described to induce netosis are dependent on ROS generation by the NADPH oxidase complex, in such a way that enzymatic inhibitors of this complex abrogate NET release2,7,8,9. Furthermore, neutrophils from chronic granulomatous disease patients are unable to release NETs, unless a ROS source is provided2,10. However, it has been demonstrated that neutrophils can release NETs without the activation of the NADPH oxidase complex. During the early/rapid vital netosis mechanism, neutrophils extrude NETs after as little as 5–15 min of activation, without affecting neutrophil viability5. Chromatin decondensation is an essential step towards netosis. Peptidyl arginine deiminase 4 (PAD4), as well as elastase and myeloperoxidase seem to be critical for NET release through the classical pathway11,12,13,14. PAD4 catalyzes histone citrullination allowing chromatin decondensation. Therefore, PAD4 knockout (KO) mice are unable to produce NETs13,14,15. Chromatin decondensation may also be accomplished by elastase and myeloperoxidase. After netosis activation, elastase migrates to the nucleus, to digest histones, a process that is further enhanced by MPO11. Therefore, elastase KO mice are netosis incapable11 and MPO deficient patients have impairment in NET release12.
We previously described that Leishmania amazonensis promastigotes induce NET release from human neutrophils, are trapped by these scaffolds and killed by the histones associated to these structures3. Here we characterize the mechanisms behind NET induction by this parasite. We investigated the participation of elastase, myeloperoxidase and PAD4 on NET formation induced in human neutrophils by L. amazonensis promastigotes. ROS involvement in NET induction was analyzed by using inhibitors of ROS/RNS (reactive nitrogen species) producing mechanisms, such as mitochondrial electron transport system, nitric oxide synthase (NOS) and xanthine oxidase. As a control, we have utilized phorbol 12-myristate 13-acetate (PMA), since it was one of the first stimuli described to induce netosis1 and a well-known cellular ROS inducer mediated by NADPH oxidase16.
Our results demonstrate that Leishmania promastigotes trigger the classical netosis, by promoting redox imbalance, with the involvement of NADPH-oxidase and NOS derived ROS/RNS, respectively. This mechanism is also dependent on PAD4 and elastase activity. Furthermore, promastigotes promoted the early/rapid, ROS-independent NET formation occurring only 10 minutes after neutrophil-parasite interaction, which is dependent of elastase, but not on PAD4.
Results
Elastase and PAD4 are involved in classical netosis induced by Leishmania
After demonstrating that Leishmania promastigotes induce NET release by human neutrophils3, we were interested to further elucidate the mechanisms involved in this process. Thus, we first assessed the role of elastase, myeloperoxidase and PAD4 on NET induction by Leishmania, by pre-treating neutrophils with their respective inhibitors. The cell permeable inhibitor of human elastase, MeOSuAAPV-CMK17, decreased netosis induction by Leishmania (Fig. 1A). A reduction of 45% and 64% was obtained upon 5 and 10 μM pretreatment with the elastase inhibitor, respectively. Similarly, elastase inhibition decreased 54% netosis induction by PMA (Fig. 1A). Due to the variability in the human donors’ response all results were presented as n fold control, but we also show the donor-to-donor variation as the amount of DNA released before and after inhibitor treatment (Fig. S1A).
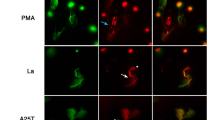
Chloroamidine and elastase inhibitor decreased netosis induced by Leishmania amazonensis (La).
Neutrophils (NØ; 2 × 106) were incubated with (A) Elastase inhibitor (E.i, 5 and 10 μM); (B) Chloroamidine (Cl-A, 12 μM) and (C) Myeloperoxidase inhibitor (MPOi, 300 nM), for 30 min and then, stimulated or not with PMA (100 nM) or promastigotes of L. amazonensis (1NØ: 0.1 La ratio) for 1 h. Following stimulation, DNA quantification was performed using PicoGreen assay kit in culture supernatants. Data normalized regarding spontaneous release of DNA representing the mean ± SEM from 17 (A), 7 (B,C) donors. *p < 0.0001 and **p < 0.05.
The involvement of PAD4 in our model was suggested by chloroamidine treatment18, which inhibited 54% and 61% of NET release by Leishmania- and PMA-activated neutrophils, respectively (Fig. 1B). Regardless the donor, chloroamidine inhibited NET release induced by Leishmania in all experiments (Fig. S1B).
The involvement of PAD4 and elastase in the netosis triggered by Leishmania was further suggested by fluorescence microscopy. Neutrophils were unable of releasing NETs when pretreated with chloroamidine and elastase inhibitor as observed by the lack of NET-DNA staining in the presence of these inhibitors (Fig. S2).
Myeloperoxidase inhibition did not affect NET formation by Leishmania-activated neutrophils (Fig. 1C). However, myeloperoxidase inhibitor19 (300 nM) reduced 36% of NET release by PMA-activated neutrophils (Fig. 1C). This result is consistent with previous reports showing that myeloperoxidase is involved in NET formation induced by PMA11,12. Of note, chloroamidine, elastase and myeloperoxidase inhibitors and diphenyleneiodonium (DPI) were not toxic to neutrophils (Table S1).
Leishmania promastigotes promote redox imbalance in neutrophils
The exposure of neutrophils to hydrogen peroxide (H2O2) induces H3 histone deimination mediated by PAD4, as previously described20. Since our results suggested the implication of histone deimination on classical netosis induced by Leishmania (Fig. 1B), we next investigated whether promastigotes would affect neutrophil redox metabolism. Thus, we followed the fluorescence increments of the redox-sensitive probe Amplex red coupled to horseradish peroxidase, which is specific for H2O2 quantification21. Our results show that, upon Leishmania or PMA-induced activation, gradually increasing levels of H2O2 were detectable within minutes after neutrophil challenge (Fig. 2A, inset). Indeed, in the presence of Leishmania, neutrophils significantly increased H2O2 production (Fig. 2A), as well as intracellular ROS levels (Fig. 2B–G). The observed redox imbalance occurs independently of parasite viability, as increased frequency of positive cells stained by dihydrorhodamine (DHR) and its fluorescence intensity, were observed in neutrophils stimulated by both viable (Fig. 2B,C) and paraformaldehyde-fixed Leishmania (Fig. 2D–G). Interestingly, DPI a flavoenzyme inhibitor22 impaired the boost in ROS formation induced by Leishmania, as evidenced by the low frequency of DHR positive cells and the fluorescence intensities as well (Fig. 2F,G). This suggests that Leishmania induced ROS production could occur through a NADPH oxidase-dependent mechanism, similarly to previous evidenced for PMA-induced neutrophil activation9.
Leishmania amazonensis (La) promastigotes activate ROS production in human neutrophils.
(A) H2O2 production was measured with Amplex Red (5 μM) after neutrophils (NØ; 2 × 106) were stimulated with PMA (100 nM) or fixed promastigotes (1 NØ: 5 La ratio) and the fluorescence recorded over 25 min of incubation, as shown on inset. Data shown as mean ± SEM of 9 independent experiments were expressed as the amount of H2O2 produced in pmol/s/2 × 106 neutrophils and as Amplex Red arbitrary units (AU, Inset). (B–G) Neutrophils (106) were incubated with DPI (32 μM) for 30 min and then stimulated with live (1NØ: 0.1La ratio) or fixed promastigotes (1NØ: 1 La ratio), incubated with DHR 123 (1.2 μM) for 20 min and analyzed by flow cytometry. Data are expressed as the percentage of DHR positive cells (B,D,F) and as the mean fluorescence intensity (MFI) (C,E,G) are shown as mean ± SEM of 23 (B,C), 4 (D,E) and 6 (F,G) different donors. Neutrophils (106) were incubated with MitoSox Red probe (2.5 μM) for 30 min at 35 °C, 5% CO2, cells were washed and stimulated with fixed promastigotes (1NØ: 1 La ratio, H,I). Neutrophils (106) were also treated with MitoSox Red (2.5 μM) and MitoTEMPO (100 μM, (J,K)). In both cases cells were then incubated for 30 min at 35 °C, 5% CO2 and analyzed by flow cytometer. Data are expressed as the mean ± SEM fluorescence intensity (MFI) of 20 (H) and 12 (J) donors. Data are expressed as the mean ± SEM percentage of MitoSox positive cells and (I,K) are shown as mean ± SEM of 11 donors. *p < 0.0001 and **p < 0.05.
Since Leishmania promastigotes were able to induce ROS production in neutrophils, we then investigated the potential contribution of mitochondria in this process. Mitochondrial ROS production was quantified by measuring the fluorescence signal of MitoSOX Red, a permeable dye that selectively targets mitochondria in live cells23. In order to determine only the neutrophil mitochondrial ROS production, the experiments were done with paraformaldehyde-fixed promastigotes. Our results show that Leishmania interaction with neutrophils induced a significantly higher frequency (Fig. 2H) and fluorescence intensity of positive MitoSOX Red stained neutrophils (Fig. 2I) when compared to control cells (20.2 vs. 12.1% positive MitoSOX Red stained cells, Fig. 2H; 9.8 vs. 6.9 AU, Fig. 2I). In order to confirm that promastigotes were stimulating mitochondrial ROS production, neutrophils were pretreated with MitoTEMPO, a mitochondrial-targeting antioxidant23. Pre-incubation of neutrophils with 100 μM MitoTEMPO before Leishmania interaction abrogated the increase in MitoSOX Red frequency of positive cells (Fig. 2J) and fluorescence intensity (Fig. 2K) induced by promastigotes (9.1 vs. 6.6% positive MitoSOX Red stained cells, Fig. 2J; 6.1vs 4.9 AU, Fig. 2K). MitoTEMPO was not toxic or induced apoptosis to neutrophils in the conditions used in the assays (Fig. S3). These data clearly demonstrate that Leishmania interaction with neutrophils induced mitochondrial ROS production.
Role of reactive oxygen species, Nitric oxide synthase, Mitochondrial ROS and xanthine oxidase on Leishmania-induced classical netosis
Following, we investigated the mechanisms responsible for ROS production that contribute to Leishmania-induced NET formation. Initially we tested the capacity of the classical NET inhibitor DPI2, to inhibit netosis induced by Leishmania promastigotes. In our experimental conditions, DPI significantly inhibited 43% of netosis induced by Leishmania. In concurrent assays using PMA as inducer, netosis inhibition was also seen after neutrophils treatment with DPI (Fig. 3A). NET-released after parasite stimulus varied among donors, but DPI significantly decreased netosis induced by Leishmania as observed by reduced NET-DNA concentration released (Fig. S1D) and by fluorescence microscopy (Fig. S2). Likewise, pretreatment of neutrophils with apocynin, which inhibits NADPH oxidase24, significantly decreased NET release induced by Leishmania (Fig. S1E). Altogether, our results suggest that Leishmania induced netosis requires, at least partially, ROS derived from a functional NADPH oxidase complex.
ROS and RNS role in NET release induced by Leishmania amazonensis (La).
Neutrophils (NØ; 2 × 106) were incubated with (A) DPI (32 μM), (B) L-NAME (1 mM), (C,D) MitoTempo (100 μM) and (E) Allopurinol (1, 2 and 4 mM) for 30 min and then stimulated or not with promastigotes (1 NØ: 0.1La ratio) or PMA (100 nM) for 1 h, followed by DNA quantification using PicoGreen assay kit in culture supernatants. Data were normalized according spontaneous release of NET-DNA representing the mean ± SEM from 22 (A), 12 (B), 7 (C), 11 (D), 10 (E) and 11 (F) donors. *p < 0.0001 and **p < 0.05.
Neutrophils produce and release nitric oxide (NO) spontaneously or following activation and both isoforms of NO synthase (NOS), inducible and constitutive, have been purified from human neutrophils25,26. To assess NO participation on Leishmania induced netosis we treated neutrophils with L-NAME, a classical NOS inhibitor27. Our results showed that L-NAME significantly inhibited 33.5% of NET-release stimulated by Leishmania and 27% by PMA (Fig. 3B).
Although mitochondria seems to contribute little to bulk oxygen consumption in neutrophils28,29,30, interference on electron transport system (ETS) at complexes I31 and III32 cause significant increase on ROS generation in these cells. Since Leishmania promastigotes were able to induce mitochondrial ROS production in neutrophils (Fig. 2), we investigated the potential contribution of this source for netosis. Scavenging mitochondrial ROS caused no apparent effect on both Leishmania (Fig. 3C) and PMA-induced netosis (Fig. 3D). These data demonstrate that despite Leishmania induced neutrophil mitochondrial ROS production, parasite-induced netosis occurs independently of this process. No toxicity was observed for neutrophils nor for parasites by MitoTempo at the concentrations used (Table S1, Fig. S3).
Next, we tested if xanthine oxidase could modulate netosis pretreating neutrophils with its inhibitor, allopurinol33. Our results demonstrated that allopurinol did not inhibit NET release induced by parasites (Fig. 3E). Interestingly, inhibition of xanthine oxidase (Fig. 3E) or myeloperoxidase (Fig. 1C) caused no effect on Leishmania-induced netosis, indicating the involvement of selective redox-dependent mechanisms in this process. Despite this general trend, inhibition of myeloperoxidase decreased Leishmania-induced NET release in some donors (Fig. S1C). Noteworthy, NET release promoted by PMA was significantly reduced (34%) by myeloperoxidase inhibitor, in agreement with previous evidence in the literature9,12.
Leishmania promastigotes activate an early/rapid netosis mechanism
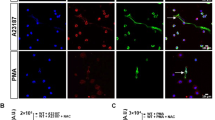
It has been shown that Staphylococcus aureus induces an early/rapid NET release, through a ROS independent pathway5. Thus, we sought to verify whether Leishmania would induce a similar mechanism. Indeed, promastigotes induced NET release after 10 min incubation with neutrophils and this early/rapid induction was not affected by DPI, which contrasts with the inhibitory role of this compound on the classical netosis testing the same donors (Fig. 4A). Additionally, these results were confirmed by immunofluorescence staining of NETs released at 10 min, which were not inhibited by DPI (Fig. S4). Interestingly, Leishmania induced ROS production detected by the frequency of DHR positive cells and by its fluorescence intensity at this early time point was significantly decreased by DPI treatment (Fig. 4B,C). The percentage of cells undergoing early NET release was 29% and increased to 41% after 1 h post stimuli (Fig. S5). Additionally, Amplex red poorly stained 10 min NETs, but strongly labeled 1 h NETs (Fig. S6), showing a higher oxygen peroxide content after 1 h of NET induction by Leishmania. This early/rapid NET release mechanism was inhibited by the elastase inhibitor but, differently from the classical netosis, was unaffected by chloroamidine (Fig. 4D). NETs formed at 10 min and 1 h were equally stained for histone H3 citrullination, but the addition of chloroamidine lowered the histone citrullination labeling only after 1 h of NET induction (Fig. S7). Because the chloroamidine inhibition also reduced NET formation only after 1 h induction (Fig. 1B), we could suggest that the contribution of PAD4 activity to NET release is relevant only during classical netosis. Furthermore, we found that the elastase activity detected in the early/rapid mechanism, it is not inhibited by DPI (Fig. 4E), in contrast with the decreased enzyme activity promoted by DPI in the classical netosis (Fig. 4E).
Rapid/Early netosis stimulated by Leishmania amazonensis (La) promastigotes occurs in a ROS-independent manner.
(A) Neutrophils (2 × 106) were incubated with DPI (32 μM) for 20 min and then, stimulated or not with promastigotes (1 NØ: 0.1 La ratio) for 10 min and 1 h. Following stimulation, DNA quantification of samples was performed using PicoGreen. (B,C) Neutrophils (106) treated with DPI (32 μM) for 20 min, were stimulated with promastigotes (1 NØ: 0.1La ratio) for 10 min, incubated with DHR 123 (1.2 μM) and immediately analyzed by flow cytometry. (D) Neutrophils (NØ; 2 × 106) were incubated with elastase inhibitor (Ei, 10 μM) and chloroamidine (Cl-A, 12 μM) for 20 min and then, stimulated with promastigotes (1NØ: 0.1 La ratio) for 10 min. DNA quantification was done as above. (E) Supernatants obtained from 10 min early/rapid or 1 h classical NET induction protocol were incubated with the elastase substrate, N-methoxysuccinyl-Ala-Ala-Pro-Val-7-amido-4-methylcoumarin (0.25 mM) for 30 min at 37 °C, 5% CO2 and the reaction product analyzed at 360/455 nm. (F) Leishmania killing after parasite treatment with NET-rich supernatants. Promastigotes (1 × 106) were incubated or not during 2 h with NET-rich supernatants obtained after 10 min or 1 h of stimuli. Then, parasites were stained with ethidium homodimer-1 (EthD-1) for 30 min and analyzed by flow cytometry. The positive control (dead parasites) was done with promastigotes treated with 0.1% Triton X-100, followed 3 cycles of freeze and thaw. Results are shown as the mean ± SEM of 3 independent experiments. *p < 0.0001 and **p < 0.05. (A,D) Data were normalized regarding spontaneous release of DNA representing the mean ± SEM from 7 (A) and 9 (D) different donors. (B,C) Data expressed as the percentage of DHR positive cells (B) and as the mean fluorescence intensity (MFI) (C) are shown as mean ± SEM of 7 different donors. (E) Data are expressed as the mean ± SEM of elastase activity in arbitrary units (AU) from 5 different donors. *p < 0.02 and **p < 0.05.
We further evaluated the promastigote viability after parasite treatment with NET-rich supernatants obtained by early/rapid or classical netosis and found 42% and 45% reduction in the Leishmania survival, respectively, indicating that NETs released by the early/rapid mechanism possess the same leishmanicidal efficiency as NETs released by the classical mechanism (Fig. 4F).
Discussion
We described here that L. amazonensis promastigotes induce neutrophil redox imbalance and NET release, involving two kinetically and mechanistically distinct processes: an early redox-independent, occurring only ten minutes after parasite contact, followed by a later redox-dependent process, reliant on ROS/RNS generated by NADPH oxidase complex and NO synthase activities. Also, netosis induced by these parasites requires the involvement of active neutrophil elastase and PAD4, but not MPO. Together, our data implicates the dynamic control of neutrophil redox homeostasis as an important mechanism of Leishmania-induced netosis.
One of the hallmarks of netosis is chromatin decondensation, which depends on both elastase and PAD4 activities and to an unknown mechanism mediated by MPO11,12,13,14,15. The role of elastase was evidenced by a decrease in netosis after inhibition of its enzymatic activity11. Moreover, elastase knockout mice were unable to release NETs after a Klebsiella pneumoniae infection11. We demonstrate that Leishmania-induced netosis is dependent on elastase activity, since its inhibition significantly affected NET release. Similarly, evidence from the literature have pointed out that PMA-stimulated netosis in neutrophils was also repressed by elastase inhibition11.Interestingly, the inhibition of NADPH oxidase activity by DPI produced kinetically distinct results both on elastase activity and on NET release, which may be explained by the different mechanisms that contribute to redox homeostasis at early (antioxidant levels) and later (NADPH oxidase + NO synthase) stages of interaction.
Histone citrullination by PAD4 is a fundamental step for chromatin decondensation and NET extrusion13,14,15. In this sense, we demonstrated that PAD4 inhibition reduced NET release by Leishmania-stimulated neutrophils, implicating this enzyme in parasite-induced classical netosis. Similar, PMA-stimulated netosis was also reduced by PAD4 inhibition. However, it remains elusive whether there is a direct relationship between protein citrullination, PAD4 activity and redox metabolism. Evidence from the literature demonstrates a boost in arginine conversion to citrulline when bone marrow cells were incubated in the presence of leukocyte conditioned medium and a superoxide radical generation source34. Interestingly, citrulline production in this system seems to be arginine deiminase and PAD4-independent, as their activities were detected in the assays conditions.
It has been shown that MPO synergize the effect of elastase on chromatin decondensation during netosis11. Neutrophils from MPO deficient donors were unable to release NETs when stimulated by PMA or Candida albicans12. However, the role of MPO on netosis seems to depend on the nature of the stimulus, since MPO-deficient neutrophils release NETs when stimulated with P. aeruginosa, S. aureus or E. coli, but fail to respond to PMA35. Likewise, inhibition of MPO activity in normal neutrophils had no effect on NET induction by P. aeruginosa, but decreased PMA-triggered netosis35. Interestingly, species-specificities also exist in terms of NET formation since inhibition of mice neutrophil MPO prevented Pseudomonas-induced netosis36, which is in contrast to netosis induced by the same pathogen in human neutrophils37. We demonstrated that Leishmania-induced netosis is not dependent on MPO activity, a feature that contrasts with netosis promoted by PMA and Candida albicans12, but not by other pathogens35. Interestingly, NO-mediated netosis is somehow dependent of active MPO, as pharmacological inhibition of this enzyme abrogates NET release38.This seems not to be the case in our experimental setting, since Leishmania-induced netosis is partially dependent on active NO synthase, but independent of MPO.
To address the role of ROS on Leishmania-induced netosis we investigated different sources of these species during neutrophil-promastigote interaction. Our results point out that NOS is involved in Leishmania-induced NET release, since L-NAME significantly decreased netosis by the parasite and by PMA as well. Indeed, inducible NO synthase activity is frequently detected at inflammation and infection sites and has already been demonstrated in cutaneous leishmaniasis lesion39,40,41,42. It has been reported that NO induces NET release from human neutrophils, which may also synergize with other stimuli present in the infection site, increasing netosis38 and NETs were evidenced in human cutaneous leishmaniasis biopsies3,43.
It is worth noting that, whatever the exact mechanism, Leishmania and PMA-induced classical netosis, several features were shared, as both are dependent on elastase, PAD4, NO synthase and NADPH oxidase activities and independent of mitochondrial-derived ROS production. In this regard, despite neutrophils mitochondria44 contributes little to cellular oxygen consumption29, inhibition of electron transport system at complexes I31 and III32 significantly increased ROS generation, indicating the potential of this organelle to modulate cellular redox homeostasis. Remarkably, although Leishmania interaction increased mitochondrial ROS production in human neutrophils, scavenging this ROS source had no effect on NET-release. In line with our observations, previous evidence demonstrated that interference of mitochondrial ROS generation, by the use of protonophores, caused no effect on PMA-induced netosis9. Evidences presented here indicate that netosis induced by Leishmania require core specific neutrophil processes, regardless the stimuli, which do not involve mitochondrial-derived ROS.
The potential contribution of xanthine oxidase on Leishmania-induced netosis as another source of cellular ROS was investigated. By using the classical xanthine oxidase inhibitor allopurinol, we observed that it caused no effect on NET production stimulated by the parasite.
Considering that the contribution of NADPH oxidase-derived ROS to the classical netosis has been largely demonstrated in the literature4,45, we tested this source of ROS, initially using pharmacological inhibitors. In our model, the association between Leishmania-induced classical netosis and ROS production by NADPH oxidase was suggested, since pretreatment of neutrophils with apocynin and DPI, inhibited NET extrusion induced by the promastigotes. Contrarily, it has been reported that L. donovani, a species that cause visceral leishmaniasis, induces netosis in a ROS-independent way46. This discrepancy with our results could be due to the different species of the parasite assayed or, most likely, because of the higher parasite-to-neutrophil ratio used in the L. donovani study. In our model, netosis inhibition by DPI was not observed when a one parasite per neutrophil ratio was assayed (data not shown).
Although the majority of NET inducers require ROS generation by NADPH oxidase, there are reports of ROS-independent netosis induced by Staphylococcus aureus, Candida albicans, uric acid, MIP-2 and ionomycin5,35,47,48,49. Moreover, netosis induction on carp granulocytes rely on ROS production when stimulated by PMA and polyinosinic:polycytidylic acid, but is ROS-independent when lipopolysaccharide was the stimulus50. It has also been described that S. aureus induces NET release by an alternative mechanism, which is ROS independent and occurs in a rapid/early period of time5,6. Interestingly, we observed that only 10 minutes after Leishmania interaction, neutrophils promote ROS generation by NADPH oxidase activity and NET release. However, early Leishmania-induced NET release is independent of ROS generation since treatment with DPI caused no effect on early NETs formation, contrasting to classical netosis.
It has been already demonstrated that NETs are toxic to several microorganisms, such bacteria and fungi1,4,51 and we also reported that L.amazonensis promastigotes induce and are killed by NETs released by the presently designated classical mechanism6. In this work we reveal that NETs released by early/rapid mechanism present the same leishmanicidal efficiency as those extruded by the classical mechanism, indicating that NETs are able to reduce parasite survival regardless their induction mechanism.
Similarly to NET release stimulated by S. aureus, our present results demonstrated that Leishmania could trigger the two types of netosis described: (i) an early/rapid ROS-independent and (ii) a late ROS-dependent mechanism6. Thus, Leishmania triggers netosis by a ROS-dependent pathway after about 1 hour, as well as by a ROS-independent mechanism occurring in 10 minutes after initial contact. Conceivably, redox imbalance promoted by Leishmania-neutrophil interaction would contribute to improve parasite trapping by NET-released DNA and their subsequent killing by neutrophil histones3.
Based on the data presented here, it is conceivable that promotion of neutrophil ROS formation, through the activation of pro-oxidant mechanisms, may improve parasite killing in vivo. Further studies are required to better understand how neutrophil redox mechanisms triggered by Leishmania induced NET formation, the dynamics of this process and their consequences for the host immune system and potential as therapeutic target.
Methods
Neutrophil purification
after informed consent from donors, blood was obtained and neutrophils were isolated by density gradient centrifugation (Histopaque; Sigma) followed by hypotonic lysis of erythrocytes. Purified neutrophils (≥95% of the cells) were re-suspended in RPMI 1640 medium (Sigma) and kept on ice until use. Experiments with human cells were performed in full compliance with the guidelines of the Research Ethics Committee of the Hospital Universitário Clementino Fraga Filho (Comite de Ética em Pesquisa (CEP), Universidade Federal do Rio de Janeiro, Brazil) and approved under the number 055-15.
Parasites
Leishmania amazonensis (MHOM/BR/75/Josefa) was maintained in Schneider Insect’s medium (Sigma) supplemented with 10% heat inactivated fetal calf serum and 40 μg/ml of gentamicin at 26 °C. Stationary phase promastigotes of 5–6 day cultures were collected, washed three times in PBS and resuspended in RPMI for further use. For some experiments promastigotes were fixed in 4% paraformaldehyde for 1 h, room temperature, extensively washed with PBS (20 ml, 4 times, 2760 g) and resuspended in RPMI.
NETs Inhibition Assays
Neutrophils (2 × 106, 200 μl) were incubated with the following inhibitors for 30 min at 35 °C, 5% CO2: diphenyleneiodonium (DPI; 32 μM; Sigma), Apocynin (APO; 1 μM; Sigma), MitoTEMPO (100 μM; Santa Cruz Biotech), N (G)-nitro-L- arginine methyl ester (L-NAME; 1 mM, Sigma), chloroamidine (Cl-A; 12 μM; Cayman Chemical), elastase inhibitor III (E.i; 5 or 10 μM, MeOSuc-Ala-Ala-Pro-Val-CMK; Calbiochem) or myeloperoxidase inhibitor-1 (MPOi; 300 nM; Calbiochem). Next, stimuli were added (promastigotes at 0.1 parasite: 1 neutrophil ratio or PMA (100 nM; Merck) and the culture incubated at 35 °C, 5% CO2 for 1.5 h. Supernatants were collected for DNA quantification using dsDNA Picogreen kit (Invitrogen), as described3. Early NET induction was performed as above except that neutrophils were incubated with the parasites for only 10 min. ROS-dependency of this early mechanism was carried out by pre-treating neutrophils with DPI (32 μM) for 20 min at 35 °C, 5% CO2, before adding the promastigotes. The role of elastase and PAD4 in the early mechanism was also evaluated treating neutrophils as above with chloroamidine and elastase inhibitor.
Recovery of NET-rich supernatants
Neutrophils (2 × 106, 200 μL) were incubated with Leishmania at 0.1 promastigotes: 1 neutrophil ratio during 10 min or 1 h at 35 °C, 5% CO2. Then, NET-rich supernatants were collected after cell culture centrifugation at 400 g for 10 min, followed by a second supernatant centrifugation at 2760 g for 20 min, to remove parasites. NET-rich supernatants were used immediately after production.
Parasite survival assay
Promastigotes (106, 200 μL) were incubated with NET-rich supernatants during 2 h at 35 °C, 5% CO2 and cell viability was assessed by treating parasites with 4 μM ethidium homodimer-1 (EthD-1) staining solution for 30 min, according to the manufacturer’s instructions (Molecular Probes). Promastigotes killed by 0.1% Triton X-100 (Sigma) treatment, followed by 3 cycles of freeze and thaw, served as positive control. Data were collected in a FACSCalibur flow cytometer and analyzed with Summit v4.3 software.
ROS Production
Neutrophils (106, 200 μL) were pre-incubated or not with DPI (32 μM) for 30 min at 35 °C, 5% CO2. Afterwards, dihydrorhodamine123 (DHR, 1.2 μM, Sigma) was added and neutrophils were stimulated with promastigotes (105) or with PMA (100 nM), for 15 min at 35 °C, 5% CO2. The fluorescence intensity of individual cells was analyzed with a FACSCalibur flow cytometer. Data analyses were performed with Summit v4.3 software.
Quantification of hydrogen peroxide production rate
H2O2 production by neutrophils (2 × 106, 200 μL), was measured with Amplex Red (Invitrogen) after stimulation with PMA (100 nM) or fixed promastigotes (107). H2O2 production was assessed by monitoring resorufin fluorescence due to the oxidation of 5 μM Amplex Red in the presence of 20 mg/mL horseradish peroxidase (Sigma), in the buffer: 125 mM NaCl, 3 mM KCl, 2 mM CaCl2, 1.25 mM NaH2PO4, 2 mM MgCl2, 20 mM HEPES. The rate of Amplex Red oxidation was recorded at room temperature using a Cary Eclipse spectrofluorimeter (Varian) adapted with a continuous stirring device, operating at excitation and emission wavelengths of 530 nm and 590 nm, respectively. After each measurement, a standard curve of H2O2 reagent grade (Sigma) was performed.
Mitochondrial ROS production
Neutrophils (106, 200 μL) were pre-incubated with MitoSox-Red (2.5 μM, Invitrogen) for 30 min at 35 °C, 5% CO2. Next, cells were washed and stimulated with fixed promastigotes (106) for 30 min at 35 °C, 5% CO2. Control experiments were carried out with neutrophils pre-incubated with MitoTEMPO (100 μM) and further challenged with promastigotes in the same conditions as described above. The optimum concentration of modulators of cellular ROS production and mitochondrial function were determined by titration of the individual compounds against human neutrophils in separate experiments. The fluorescence intensity of individual cells was analyzed by flow cytometry in a FACSCalibur flow cytometer. Data analyses were performed on Summit v4.3 software.
Immunofluorescence
Neutrophils (105, 300 μL) adhered to 0.001% poly-L-lysine-coated slide were incubated with or without inhibitors, cultured with promastigotes (105) for 10 min or 1 h as described above and fixed in 4% paraformaldehyde. NETs were visualized in a Zeiss Axioplan microscope after staining with propidium iodide (PI, 400 nM, Invitrogen). Labeling of citrullinated H3 histone was done with anti-Histone H3 (1:800; citrulline R2 + R8 + R17; AB5103, Abcam), for 1 h, followed by secondary antibody goat-anti-rabbit conjugated to Alexa 488 (1:800, Molecular Probes) together with DAPI (10 μg/mL, Sigma) for 30 min. To visualize netosis and peroxide formation, neutrophils (105) and promastigotes (104) were prepared alive in the presence of Amplex Red (5 μM, Molecular Probes) and Sytox green (0.5 μM, Invitrogen) and photographed after 10, 40 or 60 min of incubation. Images were taken using Leica DMI 6000 microscope.
Elastase activity assay
Supernatants (25 μL) obtained from early NET induction protocol were incubated with the elastase substrate, N-methoxysuccinyl-Ala-Ala-Pro-Val-7-amido-4-methylcoumarin (0.25 mM, Sigma) in buffer (50 mM HEPES, 100 mM NaCl and 0.01% Triton X-100) for 30 min at 37 °C, 5% CO2 protected from light. The reaction product was analyzed at 360/455 nm.
Apoptosis assessment
Neutrophils (106, 200 μL) were suspended in Annexin V (AnV) binding buffer (10 mM HEPES, 150 mM NaCl, 2.5 mM CaCl2) at pH 7.2, treated with MitoTEMPO (100 μM) for 30 min at 35 °C, 5% CO2 and then incubated at room temperature for 15 min with AnV-FITC (Molecular Probes) as indicated by the manufacturer. Apoptotic neutrophils were obtained by exposing cells to UV light for 2 h. Cells were analyzed by flow cytometry using a FACSCalibur flow cytometer.
Cytotoxicity of inhibitors to neutrophils and parasites
Neutrophils (2 × 106, 200 μL) were incubated with the different inhibitors for 2 h in the same conditions used for the assays and supernatants were collected for cell viability evaluation using the CytoTox® kit (Promega). Tween lysed neutrophils and purified lactate dehydrogenase were used as positive controls. Promastigotes (106) were incubated for 1 h with supernatants collected from neutrophils treated with the different inhibitors in the same conditions used for the assays. Then, PI (100 μg/ml; Sigma) was added immediately before reading on a FACSCalibur flow cytometer.
Quantification of neutrophils nuclei morphology
Neutrophils (105, 300 μL RPMI) were seeded on 0.001% poly-L-lysine-coated glass coverslip inside a 24 well plate and incubated with L. amazonensis promastigotes (104) for 10 and 60 min. Cultures were fixed with 4% paraformaldehyde and stained with propidium iodide (PI, 400 nM, Invitrogen) for 10 min and gently washed twice with PBS. Images were captured using a Zeiss Axioplan-2 microscope (Oberkochen, Germany) equipped with a Color View XS digital video camera. The nuclear shape (lobulated or decondensed) of 150–400 cells from three different donors was counted using Image J 1.46r software (National Institutes of Health) and the results shown as percentage of condensed or decondensed (netosis) neutrophils.
Statistical Analyses
Data were presented as mean ± SEM values for at least 4 different replicates of independent experiments. D´Agostino and Pearson normality tests were done for all values to assess their Gaussian distribution. Comparisons between groups were done by one-way ANOVA and a posteriori Tukey’s test for pair-wise comparisons. When appropriate, unpaired Student’s t-tests or Mann-Whitney´s test were employed. Differences of p < 0.05 were considered to be significant. When Gaussian distribution was achieved, outlier values were excluded by performing the Grubbs’ test using the online tool available at http://graphpad.com/quickcalcs/Grubbs1.cfm. All graphs and analyses were carried out by using the GraphPad Prism software version 5.00 for Windows (GraphPad Software, USA).
Additional Information
How to cite this article: Rochael, N. C. et al. Classical ROS-dependent and early/rapid ROS-independent release of Neutrophil Extracellular Traps triggered by Leishmania parasites. Sci. Rep. 5, 18302; doi: 10.1038/srep18302 (2015).
References
Brinkmann, V. U. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Fuchs, T. A. et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176, 231–241 (2007).
Guimarães-Costa, A. B. et al. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc Natl Acad Sci 106, 6748–6753 (2009).
Guimarães-Costa, A. B. et al. ETosis: A microbicidal mechanism beyond cell death. J Parasitol Res 2012, 929743 (2012).
Pilsczek, F. H. et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol 185, 7413–7425 (2010).
Yipp, B. G. & Kubes, P. NETosis: how vital is it? Blood 122, 2784–2794 (2013).
Lim, M. B. et al. Rac2 is required for the formation of neutrophil extracellular traps. J Leukoc Biol 90, 771–776 (2011).
Yost, C. C. et al. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood 113, 6419–6427 (2009).
Kirchner, T. et al. The impact of various reactive oxygen species on the formation of neutrophil extracellular traps. Mediators Inflamm 250, 1–10 (2012).
Bianchi, M. et al. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood 114, 2619–2622 (2009).
Papayannopoulos, V. et al. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 191, 677–691 (2010).
Metzler, K. D. et al. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood 117, 953–959 (2011).
Wang, Y. et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 184, 205–213 (2009).
Li, P. et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med 207, 1853–1862 (2010).
Hemmers, S. et al. PAD4-mediated neutrophil extracellular trap formation is not required for immunity against influenza infection. PLoS One 6, 1–10 (2011).
Cox, J. A. et al. Activation of the human neutrophil nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase by protein kinase C. J Clin Invest 76, 1932–1938 (1985).
Stein, R. L. & Trainor, D. A. Mechanism of inactivation of human leukocyte elastase by a chloromethyl ketone: kinetic and solvent isotope effect studies. Biochem 25, 5414–5419 (1986).
Luo, Y. et al. Inhibitors and inactivators of protein arginine deiminase 4: functional and structural characterization. Biochem 45, 11727–11736 (2006).
Kettle, A. J. et al. Mechanism of inactivation of myeloperoxidase by 4-aminobenzoic acid hydrazide. Biochem J 321, 503–508 (1997).
Neeli, I., Khan, S. N. & Radic, M. . Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol 180, 1895–1902 (2008).
Votyakova, T. V. & Reynolds, I. J. Delta Psi(m)-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem 79, 266–277 (2001).
O’Donnell, B. V. et al. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J 290, 41–49 (1993).
Dikalova, A. E. et al. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107, 106–116 (2010).
Petrônio, M. S. et al. Apocynin: chemical and biophysical properties of a NADPH oxidase inhibitor. Molecules 18, 2821–2839 (2013).
Wright, C. D. et al. Generation of nitric oxide by human neutrophils. Biochem. Biophys Res Commun 160, 813–819 (1989).
Bryant, J. L. et al. Copurification of 130 kd nitric oxide synthase and a 22 kd link protein from human neutrophils. Biochem Biophys Res Commun 189, 558–563 (1992).
Rees, D. D. et al. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro. Br J Pharmacol. 101, 746–752 (1990).
Geering, B. & Simon, H. U. Peculiarities of cell death mechanisms in neutrophils. Cell Death Differ 18, 1457–1469 (2011).
Chacko, B. K. et al. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes and neutrophils and the oxidative burst from human blood. Lab Invest. 93, 690–700 (2013).
Azevedo, E. P. et al. A Metabolic Shift toward Pentose Phosphate Pathway Is Necessary for Amyloid Fibril- and Phorbol 12-Myristate 13-Acetate-induced Neutrophil Extracellular Trap (NET) Formation. J Biol Chem. 290, 22174–22183 (2015).
Zmijewski, J. W. et al. Mitochondrial respiratory complex I regulates neutrophil activation and severity of lung injury. Am J Respir Crit Care Med 178, 168–179 (2008).
Zmijewski, J. W. et al. Participation of mitochondrial respiratory complex III in neutrophil activation and lung injury. Am J Physiol Lung Cell Mol Physiol 296, 624–634 (2009).
Sagor M. A. et al. Xanthine Oxidase Inhibitor, Allopurinol, Prevented Oxidative Stress, Fibrosis and Myocardial Damage in Isoproterenol Induced Aged Rats. Oxid Med Cell Longev. 2015, 478039 (2015)
Schneider, E. et al. A new enzymatic pathway of citrullinogenesis in murine hemopoietic cells. Biochem Biophys Res Commun 144, 829–835 (1987).
Parker, H. et al. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J Leukoc Biol 92, 841–849 (2012).
Parker, H. & Winterbourn, C. C. Reactive oxidants and myeloperoxidase and their involvement in neutrophil extracellular traps. Front Immunol 21, 424–434 (2013).
Akong-Moore, K. et al. Influences of chloride and hypochlorite on neutrophil extracellular trap formation. PLoS One 7, e42984 (2012).
Patel S. et al. Nitric oxide donors release extracellular traps from human neutrophils by augmenting free radical generation. Nitric Oxide 22, 226–34 (2010).
Arevalo, I., Ward, B. & Matlashewski, G. Detection of iNOS gene expression in cutaneous leishmaniasis biopsy tissue. Mol. Biochem. Parasitol 121, 145–147 (2002).
Atik, E., Kuk, S. & Inandi, T. Diagnostic approach and significance of inducible nitric oxide positivity in human cutaneous leishmaniasis caused by Leishmania tropica. Int J Dermatol 46, 273–277 (2007).
Palmeiro, M. R. et al. Comparative study of the in situ immune response in oral and nasal mucosal leishmaniasis. Parasite Immunol 34, 23–31 (2012).
Qadoumi, M. et al. Expression of inducible nitric oxide synthase in skin lesions of patients with American cutaneous leishmaniasis. Infect Immun 70, 4638–4642 (2002).
Morgado F. N. et al. Are Neutrophil Extracellular Traps Playing a Role in the Parasite Control in Active American Tegumentary Leishmaniasis Lesions? PLoS One. 10, e0133063 (2015).
Maianski, N. A. et al. Functional characterization of mitochondria in neutrophils: a role restricted to apoptosis. Cell Death Differ 11, 143–153 (2004).
Branzk, N. & Papayannopoulos, V. Molecular mechanisms regulating NETosis in infection and disease. Semin Immunopathol 35, 513–530 (2013).
Gabriel, C. et al. Leishmania donovani promastigotes evade the antimicrobial activity of neutrophil extracellular traps. J Immunol 185, 4319–4327 (2010).
Byrd, A. S. et al. An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. J Immunol 190, 4136–4148 (2013).
Arai, Y. et al. Uric acid induces NADPH oxidase-independent neutrophil extracellular trap formation. Biochem Biophys Res Commun 443, 556–561 (2014).
Farley, K. et al. A serpinB1 regulatory mechanism is essential for restricting neutrophil extracellular trap generation. J Immunol 189, 4574–4581 (2012).
Pijanowski, L. et al. Carp neutrophilic granulocytes form extracellular traps via ROS-dependent and independent pathways. Fish Shellfish Immunol 34, 1244–1252 (2013).
Urban, C. F. et al. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol 8, 668–676 (2006).
Acknowledgements
The authors thank the Hemotherapy Service of Hospital Clementino Fraga Filho (UFRJ) for buffy coats. This work was supported by Ministério Ciência e Tecnologia/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ). We thank the NIH Fellows Editorial Board for the edition of this manuscript.
Author information
Authors and Affiliations
Contributions
E.M.S. and M.F.O. designed the study; N.C.R., A.B.G.-C., M.T.C.N., T.S.V., M.P.O. and L.F.G.S. performed the experiments. E.M.S., N.C.R., A.B.G-C. and M.F.O. wrote the manuscript. All authors read and approved the final version of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Rochael, N., Guimarães-Costa, A., Nascimento, M. et al. Classical ROS-dependent and early/rapid ROS-independent release of Neutrophil Extracellular Traps triggered by Leishmania parasites. Sci Rep 5, 18302 (2016). https://doi.org/10.1038/srep18302
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep18302
This article is cited by
-
Neutrophil extracellular traps formation: effect of Leishmania major promastigotes and salivary gland homogenates of Phlebotomus papatasi in human neutrophil culture
BMC Microbiology (2024)
-
Moonlighting chromatin: when DNA escapes nuclear control
Cell Death & Differentiation (2023)
-
Trichomonas gallinae induces heterophil extracellular trap formation in pigeons
Parasitology Research (2023)
-
Characteristics and Role of Neutrophil Extracellular Traps in Asthma
Inflammation (2022)
-
Innate immune response in bovine neutrophils stimulated with Mycoplasma bovis
Veterinary Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.