-
PDF
- Split View
-
Views
-
Cite
Cite
Stan Deresinski, Principles of Antibiotic Therapy in Severe Infections: Optimizing the Therapeutic Approach by Use of Laboratory and Clinical Data, Clinical Infectious Diseases, Volume 45, Issue Supplement_3, September 2007, Pages S177–S183, https://doi.org/10.1086/519472
Close - Share Icon Share
Abstract
The increasingly daunting problem of antimicrobial resistance has led to an intense focus on optimization of antibiotic therapy, with simultaneous goals of improving patient outcomes and minimizing the contribution of that therapy to making the available antibiotics obsolete. Although even appropriate antibiotic therapy drives resistance, inappropriate therapy may also have adverse effects on the individual patient, as well as on the bacterial ecology. Recent research has validated the benefit of intelligent utilization of both microbiological data and clinical assessment in the empirical selection of initial broad-spectrum therapy and in further guidance of therapeutic decisions throughout the course of illness by use of a systems approach. Thus, the optimal approach to the critically ill patient with infection involves the initiation of aggressive broad-spectrum empirical therapy followed by timely responses to microbiological and clinical results as they become available. An appropriate response to this information often involves de-escalation of therapy or even its discontinuation.
Antimicrobial resistance is strongly associated with adverse patient outcomes and increased resource utilization [1]. The effective clinician in today's hospital environment must utilize all available laboratory and clinical data in the selection of the optimal antibiotic therapy for the critically ill patient. Antibiotics must, however, be utilized in a manner that ensures not only a maximally favorable outcome for the individual patient but, also, the minimization of subsequent antimicrobial resistance [2]. Each antibiotic use, whether appropriate or inappropriate, affects the bacterial ecology by exerting selective pressure and thereby driving resistance. Thus, all antibiotic use has potential public health consequences and, in this way, differs from the use of all other classes of pharmaceutical agents.
When initially faced with a patient with a serious infection, the clinician most often lacks specific knowledge of the etiologic pathogen and must choose antibiotics empirically. As the microbiological data and evolving clinical information become available, antibiotic therapy must be appropriately adjusted, often allowing a narrowing of its spectrum. Such de-escalation of therapy, along with the ultimate de-escalation (i.e., discontinuation of therapy as soon as maximum benefit has been achieved) and assurance of the heterogeneity of antibiotic use, allows for the establishment of balance between the competing tensions of the individual patient and the public health consequences of antibiotic use. The implementation of these guidelines will provide benefit to clinicians and institutions, but, most of all, it will benefit patients.
Current understanding has allowed the development of a series of simple principles of antibiotic therapy for the critically ill patient. Perhaps the most important principle is the understanding that any delay in the initiation of adequate antibiotic therapy is potentially lethal. In addition, inappropriately prolonged antibiotic therapy may adversely affect both the individual patient and the more general bacterial ecology. Multiple studies have demonstrated that survival is significantly improved when the initial choice of antibiotics is “appropriate”, defined as indicating that all isolated pathogens are susceptible to ⩾1 of the administered antibiotics [3]. Considered more broadly, however, both empirical and definitive antibiotic therapy, to be considered appropriate, require timely initiation, administration in appropriate dosages consistent with pharmacokinetic and pharmacodynamic (PK/PD) information, and appropriate alteration of therapy in response to clinical responses and microbiological data as they become available.
Among patients with health care—associated pneumonia, inadequate antimicrobial therapy is most commonly associated with infection with resistant strains of Pseudomonas aeruginosa, Staphylococcus aureus, Acinetobacter species, Klebsiella pneumoniae, and Enterobacter species [1]. An important predictor of the likelihood of inappropriate therapy is, however, the local prevalence of resistance. As a consequence, clinicians must take into account institution-specific and, if available, unit-specific epidemiological profiles and antimicrobial susceptibility patterns. Taking into account the prevalence of resistant organisms, such as methicillin-resistant S. aureus (MRSA), in the community is of critical importance.
Importance of Rapid Initiation of Appropriate Antibiotic Therapy
The impact of the choice of antibiotics is most apparent in patients who are severely ill. The interaction of appropriate therapy, severity of illness, and outcome was confirmed in a retrospective study of 142 patients with ventilator-associated pneumonia [4]. Empirical antibiotic therapy was judged to have been appropriate in 44% of patients on day 0 and in 92% of patients on day 2 [4]. A statistically significant increase in mortality rate was associated with inappropriate therapy only among patients with a high severity of illness, as judged by their Logistic Organ Dysfunction (LOD) score [4].
There is also clear evidence that, in the management of patients with severe infection, not only must the chosen antibiotic regimen be appropriate, but its administration must be promptly initiated. Kumar et al. [5] retrospectively studied the impact of delays in initiation of appropriate antimicrobial therapy in 2154 patients with septic shock and found a strong correlation between delays in initiating antibiotic therapy and in-hospital mortality (adjusted odds ratio [OR], 1.119 deaths/1-h delay; P < .0001). The time to initiation of appropriate antibiotic therapy was the strongest predictor of survival (figure 1). If an effective antibiotic was administered within the first hour of documented hypotension, the survival rate was 79.9%; each 1-h delay over the next 6 h decreased average survival rates by 7.6%. Notably, only 50% of patients received appropriate treatment during the first 6 h [5].
Early initiation of appropriate antibiotic therapy for septic shock and survival. The survival fraction is the fraction of patients surviving to hospital discharge after receiving effective therapy initiated within the given time interval. The cumulative effective antimicrobial fraction is the cumulative fraction of patients having received effective antimicrobials at any given time point. Adapted from Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34(6):1589–96 [5] (with permission from Lippincott Williams & Wilkins).
There are multiple steps involved between patient contact and antibiotic administration, and multiple reasons have been identified for delays in the administration of antibiotic therapy. These delays underscore the need for a systematic approach to the management of patients with serious infections. Reasons for delay were highlighted in a retrospective study by Iregui et al. [6] in which 33 (30.8%) of the 107 patients with ventilator-associated pneumonia had a delay in initiation of appropriate antibiotic therapy of ⩾24 h. A delay in initiation of appropriate antibiotic therapy of ⩾24 h was associated with a significantly greater hospital mortality rate (69.7% vs. 28.4%; P < .01) [6]. As in other studies, infection with an antibiotic-resistant pathogen was significantly associated with a longer delay in initiation of appropriate antibiotic therapy (P = .019), but additional contributing factors were delays in writing antibiotic orders, as well as subsequent delays in the administration of the antibiotics.
The importance of early initiation of appropriate antibiotic therapy was confirmed in an analysis of patients with sterile-site MRSA infections [7]. In this retrospective analysis of 549 patients treated over a 3-year period through the end of 2004, appropriate antibiotic therapy initiated within the first 24 h after collection of a culture-positive specimen was correlated with a significantly higher survival rate (33.4% vs. 22.0% among those who did not receive appropriate antibiotics; P = .015). of note is that all survivors received an appropriate antibiotic within 6 h of the availability of the culture result. An instructive observation was that the likelihood of administration of inappropriate antibiotic therapy was significantly higher among patients whose onset of infection occurred <48 h after admission to the hospital than among patients with later onset of infection, despite the fact that 88.4% of patients with early onset of infection had risk factors for health care—associated infection (see below for risk factors), indicating that clinicians failed to recognize these risks.
Also of note is that the importance of early initiation of appropriate antimicrobial therapy is not limited to bacterial infections. Recent studies have found similar improved survival among patients with candidemia who received antifungal therapy in a timely fashion [8, 9].
Risk Factors For Infection With Multidrug-Resistant (Mdr) Pathogens and Choice of Antibiotic Therapy
The guidelines of the American Thoracic Society and the Infectious Diseases Society of America for the management of health care—associated pneumonia indicate that risk factors for infection with MDR pathogens include receipt of antimicrobial therapy within the previous 3 months, current hospitalization of ⩾5 days, a high frequency of antibiotic resistance in the community or specific hospital unit, presence of immunosuppressive disease or receipt of immunosuppressive therapy, and the presence of factors defining pneumonia as being health care related [10]. These latter factors include hospitalization for ⩾2 days within the past 90 days, residence in a nursing home or extended-care facility, home infusion therapy (including antibiotics), chronic dialysis within the past 30 days, home wound care, and a family member with an MDR-associated infection [10].
The American Thoracic Society/Infectious Diseases Society of America guidelines recommend broad-spectrum combination therapy with ⩾2 antibiotics as the initial empirical therapy for patients with health care—associated pneumonia and risk factors for being infected with resistant organisms. The recommended regimens to be considered are an aminoglycoside OR an antipseudomonal fluoroquinolone plus an antipseudomonal β-lactam (a carbapenem, if extended-spectrum β-lactamase—producing pathogens or MDR Acinetobacter species are suspected) PLUS vancomycin or linezolid if MRSA is suspected [3, 10]. With regard to the treatment of MRSA pneumonia, however, the guidelines acknowledge that vancomycin may be an inferior choice. As discussed in detail by Kollef [11] elsewhere in this supplement, vancomycin has significant limitations in the treatment of infections due to S. aureus, because its activity against this pathogen is steadily decreasing [12–15]. Other therapeutic options are currently limited: daptomycin is unsuitable for use for lung infections because of inactivation by surfactant, and tigecycline remains under investigation for this purpose.
Choosing the correct antibiotics is only the start of effective antimicrobial chemotherapy. Just as important is the attention that must be paid to PK/PD principles to maximize optimal antimicrobial effects, minimize the likelihood of emergence of resistance, and minimize the potential adverse effects of therapy. Achieving PD targets has been associated with successful bacterial eradication and clinical success, as well as with a reduced risk of resistance emergence. An example of the importance of the latter can be seen in the work of Thomas et al. [16], who evaluated the relationship between antibiotic PK/PD and the outcomes in 107 patients with nosocomial lower respiratory tract infection. The results indicated that an area under the concentration-time curve/minimum inhibitory concentration (AUC/MIC) of ⩾100 was associated with a significantly lower risk of emergence of resistant organisms.
Empirical Initial Antibiotic Therapy and De-Escalation
Kollef and Micek [17] developed an algorithm to help guide empirical antibiotic therapy, as well as its subsequent de-escalation and discontinuation. Thus, in the presence of severe infection of possible bacterial etiology, specimens for microbiological analysis should be obtained and empirical broad-spectrum antibiotic therapy promptly initiated. The choice of therapy depends on host factors, including the risk for infection with antibiotic-resistant pathogens, with guidance from local susceptibility patterns. Although clinical status and response should be continually monitored, assessment at 48–72 h, a time when relevant microbiological data are generally available, is a time of major reevaluation of empirical therapy. If, at that time, the patient's condition has clinically improved and microbiological data indicate the appropriateness of the antibiotic therapy, tailoring the therapy to a narrower spectrum is indicated. This de-escalation of antibiotic therapy may, in fact, be extended to its discontinuation, if evidence at this time points away from the presence of bacterial infection [18]. If, in contrast, the patient's condition has failed to improve clinically by this time, a careful reassessment of the available microbiological data must be performed, with consideration of the possibility of infection with a resistant bacterial or nonbacterial pathogen. The evaluation must also include an assessment of the possible presence of complications, such as abscess formation, as well as of the possibility of a noninfectious cause of the patient's septic profile. Once the patient's infection is under control, attention should be paid to discontinuation of antibiotic therapy as soon as maximum benefit has been achieved [2, 18].
The benefits of de-escalation and prompt discontinuation of antibiotic therapy include cost minimization, a lessening of the risk of drug-related adverse events, and a reduction in pressure on the bacterial ecology, thereby diminishing the likelihood of emergence of resistant pathogens. Examples of the benefits of early antibiotic discontinuation include a likely decrease in the incidence of Clostridium difficile—associated diarrhea and a reduction in the incidence of superinfection with resistant bacteria and Candida organisms. A clear example of the latter benefit emerged from a study by Singh et al. [19], who evaluated the impact of a short-course (3 days) of antibiotic therapy in conjunction with a formal scoring system designed to determine the diagnosis of nosocomial pneumonia (the Clinical Pulmonary Infection Score) in 81 patients in the surgical intensive care unit (ICU) who had pulmonary infiltrates. The likelihood of an infectious cause of the infiltrates was evaluated on day 3, and patients with low Clinical Pulmonary Infection Scores were randomized to have their antibiotic therapy either discontinued or continued for a duration determined on the basis of their scores. No adverse consequences, such as increased mortality or length of stay, were associated with the discontinuation of antibiotic therapy after 3 days in patients who proved to have a low likelihood of pneumonia. However, for these low-risk patients, early discontinuation was associated with a significantly reduced risk of emergence of resistant pathogens, which occurred in only 15% of the patients versus 35% (P = .017) of those whose therapy was continued beyond 3 days.
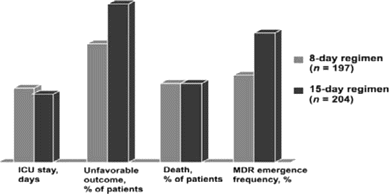
Although many of the low-risk patients in the study by Singh et al. [19] may never have had pulmonary infection at all, reducing the duration of antibiotic therapy has also proven to be an effective strategy in patients with proven bacterial pneumonia. Thus, in a randomized trial, Chastre et al. [20] demonstrated similar clinical outcomes, as well as a reduction in subsequent infection with resistant pathogens among patients with ventilator-associated pneumonia who received appropriate antibiotic therapy for 8 days, as opposed to 15 days (figure 2). A degree of caution is indicated, however, because patients infected with nonfermenting gram-negative bacilli (mostly P. aeruginosa) demonstrated a non—statistically significant trend toward an increased risk of recurrence of infection among those treated with the regimen of shorter duration.
Appropriate empirical antibiotic therapy of ventilator-associated pneumonia: 8 vs. 15 days. In a multicenter, randomized, double-blind trial involving patients with ventilator-associated pneumonia, the 8-day regimen achieved outcomes better than or equivalent to those associated with the 15-day regimen. “Unfavorable outcome” was defined as death, recurrence of pulmonary infection, or prescription of a new antibiotic for any reason. ICU, intensive care unit; MDR, multidrug resistance.
Although the concept of de-escalation seems to be simple, its systematic and timely implementation often proves difficult in practice. The experience at one university hospital ICU is instructive in this regard: although de-escalation was successfully implemented in 69% of cases in which microbiological data indicated that it was appropriate, there was a mean interval of ∼48 h from the time that the relevant microbiological data were available to the actual time of de-escalation [21]. This demonstrates the need for systematic approaches to assuring timely therapeutic de-escalation with information systems allowing real-time linkages between decision support systems, the clinical microbiology laboratory, the pharmacy, and clinicians. Even with the implementation of such systematic approaches, it is necessary that the prescribing physician make appropriate changes to therapy as the necessary information becomes available. The optimal means of achieving this last step, in particular, is likely to require the establishment of an effective antimicrobial stewardship program [22].
Systems Approach to the Optimization of Antibiotic Therapy
The necessity of formalized systematic approaches to the optimization of antibiotic therapy has become increasingly apparent. The optimal approach requires the development of a multidisciplinary team with the full support, including necessary monetary support, of an institution's administration. The Infectious Diseases Society of America has recently published evidence-based guidelines supporting the implementation of a formal program designed to improve antimicrobial use in the hospital (table 1) [22]. Their recommendations include the establishment of a multidisciplinary team whose core consists of an infectious diseases physician, an infectious diseases pharmacist, a clinical microbiologist, an information system specialist, an infection control professional, and an epidemiologist. The basic strategies that may be considered for implementation are prospective audit, with intervention as necessary, together with individualized feedback and formulary restriction with preauthorization. These strategies are not mutually exclusive. Additional elements of the program may include clinician education (in addition to the individualized real-time feedback) and the development of antibiotic use guidelines, as well as the implementation of a program of streamlining and de-escalation of therapy [22]. A frequently suggested approach, antibiotic cycling, is not positively endorsed, because of an assessment that there is insufficient evidence for its support. In fact, there may be sufficient available evidence, both theoretical and empirical, to recommend against antibiotic cycling [23–26]. In contrast, mathematical modeling and limited clinical experience indicate that a more effective approach to slowing the progression of antimicrobial resistance is to assure heterogeneity of antibiotic use [23, 27].
Infectious Diseases Society of America guidelines for antimicrobial stewardship: comprehensive multidisciplinary antimicrobial management program.
It should be noted that one commonly used approach, the establishment of a restrictive formulary, is in opposition to the heterogeneous use of antibiotics and, if strictly enforced, may actually exacerbate the problem of antibiotic resistance. Restrictive formularies are problematic for institutions with outpatient programs, because of the implementation of Medicare Part D, which necessitates the availability of medications in the program chosen by the participant. Another approach, the routine use of definitive pathogen-directed combination therapy, is appealing in theory, but data supporting a positive impact on the emergence of resistant pathogens are not available.
The effects of the implementation of some of these elements of antimicrobial stewardship were recently examined in a questionnaire study by Zillich et al. [28] that involved 448 US hospitals. The effect of 5 independent variables (dissemination of clinical practice guidelines for antimicrobial use, implementation of guidelines, use of antimicrobial-related information technology, use of decision support tools, and communication to prescribers about antimicrobial use) on antimicrobial resistance rates was analyzed [28]. The results indicated that implementation of antibiotic use guidelines by hospitals and optimization of empirical antibiotic prophylaxis were associated with lower rates of resistance (P < .01); in contrast, the other control measures were associated with greater rates of resistance, as was the use of restrictive formularies (P = .05) [28].
Prevention of Antimicrobial Resistance—Some Additional Recent Approaches
The Centers for Disease Control and Prevention have published a 12-step program designed to slow the march of antimicrobial resistance, focusing on the effective use of devices, accurate diagnoses, the careful use of antimicrobials, and prevention of pathogen transmission [29]. Highlighting the importance of infection control measures as a means of limiting infections due to antibiotic-resistant pathogens, Huang et al. [30] examined the impact of 4 infection control measures on the incidence of MRSA bacteremia in a single hospital. The use of routine surveillance cultures and patient isolation was associated with a significantly reduced incidence of MRSA bacteremia (a 75% reduction in ICU patients [P = .007] and a 40% reduction in non-ICU patients [P = .008]) [31]. The absence of a contemporaneous decrease in the incidence of methicillin-susceptible staphylococcal bacteremia was consistent with the conclusion that the decrease in MRSA infections was the result of the study intervention.
Continuing the focus on MRSA infections and infection control practices, Girou et al. [31] found that good compliance with hand hygiene practices at the bedside of the patient was a strong predictor of reduced MRSA prevalence. The relationship between hand hygiene and resistance rates was significant even after adjustment for the length of stay in the hospital and the number of hand hygiene opportunities per hour of care, which the authors suggested was a surrogate for nursing workload and, to a lesser extent, severity of infection [31].
Finally, the routine use of mupirocin ointment and chlorhexidine baths for patients with nasal carriage of MRSA was associated with a significant reduction in nosocomial MRSA infections in ICU patients [32]. From January 1999 to December 2003, ICU patients with nasal carriage of MRSA received 2% mupirocin ointment intranasally 3 times daily for 5 days and a chlorhexidine bath once daily for 3 days. A total of 2200 patients underwent screening, and 364 were found to have nasal carriage of MRSA. The rates of nosocomial MRSA infection steadily declined during the intervention period, from 8.3% in 1999 to 6.3% in 2000, 4.3% in 2001, 3.6% in 2002, and 2.8% in 2003. The difference between the rates in 1999 and 2003 was statistically significant (P = .001) [32].
Thus, it seems likely that rigorous institution of robust process-of-care improvements that include both antimicrobial stewardship and effective infection control procedures can result in a lower likelihood of bacterial resistance and improved clinical outcomes.
Conclusions
In approaching the patient with a serious infection, it is critically important that rapid initiation of effective antibiotic therapy occur, followed by de-escalation and, when indicated, prompt discontinuation of therapy, as new information becomes available. At the same time, the application of effective infection control measures provides critical complementary means of controlling antibiotic resistance. The effective implementation of these principles requires the development of multidisciplinary antimicrobial stewardship programs that assure adherence.
Acknowledgments
Supplement sponsorship. This article was published as part of a supplement entitled “Bugging the Bugs: Novel Approaches in the Strategic Management of Resistant Staphylococcus aureus Infections”, jointly sponsored by the Dannemiller Memorial Educational Foundation and Emeritus Educational Sciences and supported by an educational grant from Ortho-McNeil, Inc., administered by Ortho-McNeil Janssen Scientific Affairs, LLC.
Potential conflicts of interest. S.D. is a consultant for Schering-Plough and Ortho-McNeil, and is on the speakers' bureaus for Merck, Ortho-McNeil, Schering-Plough, Pfizer, Wyeth, and Cubist.




![Early initiation of appropriate antibiotic therapy for septic shock and survival. The survival fraction is the fraction of patients surviving to hospital discharge after receiving effective therapy initiated within the given time interval. The cumulative effective antimicrobial fraction is the cumulative fraction of patients having received effective antimicrobials at any given time point. Adapted from Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34(6):1589–96 [5] (with permission from Lippincott Williams & Wilkins).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/45/Supplement_3/10.1086_519472/1/m_45-Supplement_3-S177-fig001.gif?Expires=1716387083&Signature=McRH3TM11qGhZZWghqvrSLVohVSqSMOJeqXU6p4dFF5mM2~ke9-YWGvyG6Ee~~15z5cjMHklygQ3q-MSla9egCv56NVplK7JXgZcKk31cdapQxW-lF2u1PxiRiPnSwTqu1J~EJofl6OhPMpXj4PlN2NI03ZDjSdnLV9an0CRVc-QjcaJDs8CqlnFBMTUGoH2~JrJwV4xTwOGxAQkAgA3msR8sjC~8ipuXFPTUl9sRu3XOEV19HN9aeTyUiX0T2nmpSoG~A8Wsi-5R1A2V2TpE8Qgh8BaFc19ukaPS7K5aGTaUROXkNx6W5WsI4k~PjdIMnW8vdN3xu3IhYoqMjsyCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


Comments