-
PDF
- Split View
-
Views
-
Cite
Cite
Keun-Hyok Cho, Young-Bong Song, Ik-Sung Choi, Eun-Hee Cho, Jae-Won Choi, Young Mi Ahn, Yong Ho Roh, Seung-Hyun Nam, Bong-Seog Kim, A Phase II Study of Single-Agent Gemcitabine as a Second-Line Treatment in Advanced Non-Small Cell Lung Cancer, Japanese Journal of Clinical Oncology, Volume 36, Issue 1, January 2006, Pages 50–54, https://doi.org/10.1093/jjco/hyi213
Close - Share Icon Share
Abstract
Objective: To evaluate the efficacy and safety of the single-agent gemcitabine in advanced non-small cell lung cancer (NSCLC) as second-line chemotherapy.
Methods: Between February 2002 and November 2004, a total of 27 patients, who had previously been treated with paclitaxel and platinum as first line chemotherapy, were enrolled in the study. Patients were treated with gemcitabine (1000 mg/m2) on days 1, 8 and 15 in a 28 day cycle. The response was assessed every two cycles. Toxicities were evaluated according to common toxicity criteria (CTC).
Results: The median age was 62 (range, 46–79) years old. Among the 27 patients, 26 were male. Twenty-three patients had an ECOG performance status of 0 or 1 and four patients had a status of 2. Pathologically, 24 patients had squamous cell carcinoma and 3 had adenocarcinoma. Partial responses were observed in 15 patients. All patients were evaluated for response and toxicity. The overall response rate was 18.5% (95% confidence interval, 5–33%) and the median response duration was 17 (range, 7.4 to 49+) weeks. The median time to progression was 10 (range, 7 to 34+) weeks. The median overall survival for all patients was 38 (range, 10 to 122+) weeks. During a total of 87 cycles, granulocytopenia greater than CTC grade 2 occurred in 7%, thrombocytopenia in 1% and anemia in 24% of case. Non-hematologic toxicities were minor and easily controlled. Conclusion: This study confirms the activity and safety of the single-agent gemcitabine as a second-line therapy in pretreated patients with advanced NSCLC.
INTRODUCTION
Lung cancer is currently the leading cause of death from malignant disease in Korea (1). The results of a large meta-analysis of 52 randomized clinical trials showed conclusively that the administration of chemotherapy offers a modest but significant survival benefit compared with the best supportive care (2). Use of chemotherapy for advanced non-small cell lung cancer (NSCLC) has recently become more customary. Randomized studies showed that cisplatin-based chemotherapy and new chemotherapeutic agents result in improved quality of life compared with the best supportive care (3). Consequently, for advanced NSCLC patients with a good performance score, a platinum-based regimen is now advised.
Virtually all patients who respond initially will eventually relapse. Because of improved therapeutic results with newer agents, a growing number of patients experience a longer disease-free interval compared with the older regimens. At relapse, the patients are likely to have a good performance status and be eligible for further treatment. The role of second-line chemotherapy after initial treatment with a platinum-based regimen remains largely undefined. Results from a number of recent studies on second-line chemotherapy are challenging this view (4).
One of the most active new drugs in NSCLC is gemcitabine (2′-2′ difluorodeoxycytidine) a nucleoside analog that, as a single-agent, has shown a response rate of ∼20% in previously untreated patients (5,6). As a first line treatment, single-agent gemcitabine has been shown to have anti-tumor activity equal to that of cisplatin/etoposide with less toxicity and slightly better quality of life resulting (7). In vitro gemcitabine still shows activity in platinum resistant cell lines (8).
In view of the promising data about gemcitabine, we started a phase II study of the single-agent gemcitabine as a second-line therapy in advanced NSCLC with the aim to assess response and evaluate toxicity.
PATIENTS AND METHODS
Patient Selection
Patients were included in the study if they had a relapse or progression of proven advanced NSCLC during or prior to therapy. Radiotherapy was allowed as long as it had been completed at least 4 weeks prior to inclusion in the study, to allow the patients to recover from any side effects, and the disease had progressed outside the radiation field. All patients had to have an ECOG performance status of 0–2, one or more lesions that could be measured from each side, and adequate baseline organ function, defined as a WBC count of at least 4000/μl, a platelet count of at least 100 000/μl, a total bilirubin level of <3.0 mg/dl, serum transaminases levels of less than three times the upper limit of normal, and a serum creatinine value of <1.5 mg/dl or a creatinine clearance of >50 ml/min.
Patients with symptomatic brain metastases or other severe illnesses were excluded from the study. Written informed consent was required from each patient prior to inclusion. The protocol was approved by the institutional review board (IRB) at the Seoul Veterans Hospital of Korea.
Patient Evaluation
A complete history, physical examination, recording of performance status according to ECOG criteria, complete blood cell count with differential, serum biochemistry, urinalysis and ECG were obtained at baseline for each patient. Chest radiographs and CT scans were routinely performed in all patients. Other radiographic examinations, e.g. isotope bone scans, brain CT scan, abdominal ultrasonography and abdominal CT scan, were performed if clinically indicated.
Treatment and Dose Adjustments
Single-agent gemcitabines was administered on an outpatient basis. Gemcitabine (1000 mg/m2) was administered as a 30 min intravenous infusion on days 1, 8 and 15 of each 28 day cycle. Prophylactic antiemetics were administered on the day of chemotherapy with 5-HT3 receptor antagonists. Drug administration was delayed to a maximum of 2 weeks if there was incomplete hematological recovery on day 29 (WBC < 3000/μl and/or platelet <75 000/μl) or in the case of persistent NCI common toxicity criteria (CTC) grade 2 or more non-hematological toxicity. The dose of gemcitabine was reduced to 75% in cases where the WBC was between 3000 and 4000/μl or platelet count was between 75 000/μl and 100 000/μl, and reduced to 50% in cases where the WBC was between 2000 and 3000/μl or platelet count was between 50 000/μl and 75 000/μl. Doses were omitted in cases where the WBC had dropped to <2000/μl, platelets <50 000/μl or in cases where there was CTC grade 3 non-hematological toxicity. The cycle was repeated a maximum of six times and was stopped earlier if there was evidence of progressive disease, unacceptable toxicity or by the patient's wish. No other chemotherapy or experimental medication was permitted while the patients were on this study.
Response and Toxicity Assessment
Physical examinations, complete blood counts and biochemistry profiles were repeated every 4 weeks. Complete blood counts were measured before each gemcitabine infusion. Toxicities were graded according to CTC (version 3.0). Radiographic evaluations for tumor response, including isotopic bone scan and CT scan, were performed every two courses of chemotherapy. Tumor response was measured according to standard WHO criteria.
The response duration was measured from the date of confirmation of at least partial tumor response to the date of disease progression. Progression-free survival was measured from the commencement of therapy until the date of confirmation of disease progression. Survival was measured from the commencement of therapy until the date of death or last follow-up evaluation. After the discontinuation of treatment, patients were evaluated every 8 weeks to assess disease progression.
Method of Analysis and Sample Size
Statistical analysis was performed with the SPSS (version 10.0) statistical program. Response rates, according to the prognostic factors, were compared by Fisher's exact test. Survival curves were estimated by the Kaplan–Meier method, and the log-rank test was used to compare the difference in survival and duration of response. The logistic regression analysis was used in a multivariate model.
In this study, the estimation of sample size was as below. The Simon two-stage phase II design provided 80% power and a 0.05 level of significance overall to distinguish between the null and alternative hypotheses, where the null hypothesis (H0) is that the true overall response rate is <5% and the alternative hypothesis (H1) is that the true overall response rate is >25%. The trial would be stopped at nine patients if zero patients have responded or at 24 patients if two have responded after two treatment cycles.
RESULTS
Patient Characteristics
From February 2003 to November 2004, a total of 27 patients were enrolled in the study. The baseline characteristics of the patients treated with second-line gemcitabine are shown in Table 1. Twenty-six patients were male. The median age was 62 years (range, 46–79 years), 16 (59%) patients were older than 60 years. The ECOG performance status was 0–1 in 23 (85%) patients and 2 in 4 (15%) patients. Pathologically the majority of patients had squamous cell carcinoma (81%). One patient had stage IIIA disease, 13 had stage IIIB and 13 had stage IV disease. Five patients had received prior radiation therapy. Twenty-three patients had previously received paclitaxel and carboplatin and four patients received paclitaxel and cisplatin as their first-line treatment. A total of 87 courses of treatment were given, at a median of two courses per patients (range, 2–6 courses). The median time between the completion of first-line treatment and starting second-line gemcitabine was 20 weeks (range, 6–75 weeks).
Pretreatment characteristics of patients
| Characteristics . | No. of patients (%) . | |
|---|---|---|
| Total/evaluable | 27/27 | |
| Sex | ||
| Male/Female | 26/1 | |
| Age (years) | ||
| Median [range] | 62 [46–79] | |
| Performance status (ECOG) | ||
| 0–1 | 23 (85) | |
| ≥2 | 4 (15) | |
| Histology | ||
| Squamous cell carcinoma | 22 (81) | |
| Adenocarcinoma | 5 (19) | |
| Stage | ||
| III | 14 (52) | |
| IV | 13 (48) | |
| Treatment interval (month) | ||
| ≤6 | 15 (56) | |
| >6 | 12 (44) | |
| Previous response | ||
| Yes | 10 (37) | |
| No | 17 (63) | |
| Previous radiotherapy | 5 (19) | |
| Characteristics . | No. of patients (%) . | |
|---|---|---|
| Total/evaluable | 27/27 | |
| Sex | ||
| Male/Female | 26/1 | |
| Age (years) | ||
| Median [range] | 62 [46–79] | |
| Performance status (ECOG) | ||
| 0–1 | 23 (85) | |
| ≥2 | 4 (15) | |
| Histology | ||
| Squamous cell carcinoma | 22 (81) | |
| Adenocarcinoma | 5 (19) | |
| Stage | ||
| III | 14 (52) | |
| IV | 13 (48) | |
| Treatment interval (month) | ||
| ≤6 | 15 (56) | |
| >6 | 12 (44) | |
| Previous response | ||
| Yes | 10 (37) | |
| No | 17 (63) | |
| Previous radiotherapy | 5 (19) | |
Pretreatment characteristics of patients
| Characteristics . | No. of patients (%) . | |
|---|---|---|
| Total/evaluable | 27/27 | |
| Sex | ||
| Male/Female | 26/1 | |
| Age (years) | ||
| Median [range] | 62 [46–79] | |
| Performance status (ECOG) | ||
| 0–1 | 23 (85) | |
| ≥2 | 4 (15) | |
| Histology | ||
| Squamous cell carcinoma | 22 (81) | |
| Adenocarcinoma | 5 (19) | |
| Stage | ||
| III | 14 (52) | |
| IV | 13 (48) | |
| Treatment interval (month) | ||
| ≤6 | 15 (56) | |
| >6 | 12 (44) | |
| Previous response | ||
| Yes | 10 (37) | |
| No | 17 (63) | |
| Previous radiotherapy | 5 (19) | |
| Characteristics . | No. of patients (%) . | |
|---|---|---|
| Total/evaluable | 27/27 | |
| Sex | ||
| Male/Female | 26/1 | |
| Age (years) | ||
| Median [range] | 62 [46–79] | |
| Performance status (ECOG) | ||
| 0–1 | 23 (85) | |
| ≥2 | 4 (15) | |
| Histology | ||
| Squamous cell carcinoma | 22 (81) | |
| Adenocarcinoma | 5 (19) | |
| Stage | ||
| III | 14 (52) | |
| IV | 13 (48) | |
| Treatment interval (month) | ||
| ≤6 | 15 (56) | |
| >6 | 12 (44) | |
| Previous response | ||
| Yes | 10 (37) | |
| No | 17 (63) | |
| Previous radiotherapy | 5 (19) | |
Response and Survival
All of the patients completed at least two cycles and were evaluated for response and toxicity. After a median two cycles of chemotherapy, there were no complete responses and five partial responses, for an overall response rate of 18.5% (95% confidence interval, 5–33%). The median response duration was 17 weeks (range, 7.4 to 49+ weeks).
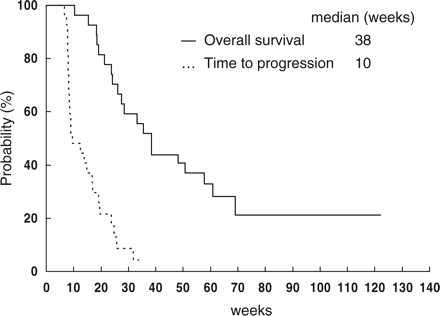
The median follow-up period was 9 months. At the time of this analysis, 17 patients were reported dead. The median progression-free survival was 10 weeks (range, 7 to 34+ weeks). The median survival time was 38 weeks (range, 10 to 122+ weeks) and 1 year survival rate was 37% (Fig. 1). The median survival duration was not reached in responding patients and was 33 weeks in non-responding patients, however the difference between the two groups was not statistically significant (P = 0.15).
Time to progression and overall survival curves of total patients.
Salvage Treatment after Second-line Gemcitabine
Fifteen patients were managed with a salvage regimen after the failure of second-line gemcitibine. Ten patients were treated with gefitinib (Iressa, an epidermal growth factor receptor inhibitor). Two patients showed a partial response with durations of 17 and 32 weeks. The median time to progression was 12 weeks in patients treated with gefitinib as a third-line therapy. Four patients received single vinorelbine and two patients received combination chemotherapy with docetaxel and cisplatin.
Prognostic Factor Analysis
A prognostic factor analysis was performed for response and survival with variable factors (Table 2), including age (≤60 versus >60 years), performance status (ECOG 0 or 1 versus 2), stage at the time of second-line therapy (stage III versus stage VI), pathological finding (adenocarcinoma versus squamous cell carcinoma), treatment interval (6 versus >6 months) and response to first-line therapy. There was no statistically significant parameter for response. By univariate analysis, only performance status was found to be of prognostic significance in terms of overall survival: median survival of 48 weeks for ECOG 0 to 1 versus 24 weeks for ECOG 2 (P = 0.02). A multivariate analysis for survival showed no parameter to be a significant prognostic factor.
Analysis of prognostic factors
| . | Response . | . | . | TTP* . | . | OS† . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | n . | % . | P . | Median (weeks) . | P . | Median (weeks) . | P . | |||||||
| Age | ||||||||||||||
| ≤60 | 13 | 8 | 9 | 29 | ||||||||||
| >60 | 14 | 29 | 0.33 | 10 | 0.40 | 44 | 0.37 | |||||||
| Performance status (ECOG) | ||||||||||||||
| 0–1 | 23 | 40 | 10 | 48 | ||||||||||
| 2 | 4 | 0 | 0.56 | 9 | 0.64 | 24 | 0.27 | |||||||
| Histology | ||||||||||||||
| SQ‡ | 22 | 14 | 9 | 38 | ||||||||||
| Adeno§ | 5 | 40 | 0.22 | 19 | 0.44 | 19 | 0.99 | |||||||
| Stage | ||||||||||||||
| III | 14 | 14 | 9 | 26 | ||||||||||
| IV | 13 | 23 | 0.65 | 17 | 0.53 | 44 | 0.21 | |||||||
| Previous response | ||||||||||||||
| Yes | 10 | 30 | 9 | 38 | ||||||||||
| No | 17 | 12 | 0.33 | 14 | 0.60 | 33 | 0.52 | |||||||
| Treatment interval (month) | ||||||||||||||
| ≤6 | 15 | 13 | 9 | 29 | ||||||||||
| >6 | 12 | 25 | 0.63 | 10 | 0.97 | 48 | 0.23 | |||||||
| . | Response . | . | . | TTP* . | . | OS† . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | n . | % . | P . | Median (weeks) . | P . | Median (weeks) . | P . | |||||||
| Age | ||||||||||||||
| ≤60 | 13 | 8 | 9 | 29 | ||||||||||
| >60 | 14 | 29 | 0.33 | 10 | 0.40 | 44 | 0.37 | |||||||
| Performance status (ECOG) | ||||||||||||||
| 0–1 | 23 | 40 | 10 | 48 | ||||||||||
| 2 | 4 | 0 | 0.56 | 9 | 0.64 | 24 | 0.27 | |||||||
| Histology | ||||||||||||||
| SQ‡ | 22 | 14 | 9 | 38 | ||||||||||
| Adeno§ | 5 | 40 | 0.22 | 19 | 0.44 | 19 | 0.99 | |||||||
| Stage | ||||||||||||||
| III | 14 | 14 | 9 | 26 | ||||||||||
| IV | 13 | 23 | 0.65 | 17 | 0.53 | 44 | 0.21 | |||||||
| Previous response | ||||||||||||||
| Yes | 10 | 30 | 9 | 38 | ||||||||||
| No | 17 | 12 | 0.33 | 14 | 0.60 | 33 | 0.52 | |||||||
| Treatment interval (month) | ||||||||||||||
| ≤6 | 15 | 13 | 9 | 29 | ||||||||||
| >6 | 12 | 25 | 0.63 | 10 | 0.97 | 48 | 0.23 | |||||||
Time to progression.
Overall survival.
Squamous cell carcinoma.
Adenocarcinoma.
Analysis of prognostic factors
| . | Response . | . | . | TTP* . | . | OS† . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | n . | % . | P . | Median (weeks) . | P . | Median (weeks) . | P . | |||||||
| Age | ||||||||||||||
| ≤60 | 13 | 8 | 9 | 29 | ||||||||||
| >60 | 14 | 29 | 0.33 | 10 | 0.40 | 44 | 0.37 | |||||||
| Performance status (ECOG) | ||||||||||||||
| 0–1 | 23 | 40 | 10 | 48 | ||||||||||
| 2 | 4 | 0 | 0.56 | 9 | 0.64 | 24 | 0.27 | |||||||
| Histology | ||||||||||||||
| SQ‡ | 22 | 14 | 9 | 38 | ||||||||||
| Adeno§ | 5 | 40 | 0.22 | 19 | 0.44 | 19 | 0.99 | |||||||
| Stage | ||||||||||||||
| III | 14 | 14 | 9 | 26 | ||||||||||
| IV | 13 | 23 | 0.65 | 17 | 0.53 | 44 | 0.21 | |||||||
| Previous response | ||||||||||||||
| Yes | 10 | 30 | 9 | 38 | ||||||||||
| No | 17 | 12 | 0.33 | 14 | 0.60 | 33 | 0.52 | |||||||
| Treatment interval (month) | ||||||||||||||
| ≤6 | 15 | 13 | 9 | 29 | ||||||||||
| >6 | 12 | 25 | 0.63 | 10 | 0.97 | 48 | 0.23 | |||||||
| . | Response . | . | . | TTP* . | . | OS† . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | n . | % . | P . | Median (weeks) . | P . | Median (weeks) . | P . | |||||||
| Age | ||||||||||||||
| ≤60 | 13 | 8 | 9 | 29 | ||||||||||
| >60 | 14 | 29 | 0.33 | 10 | 0.40 | 44 | 0.37 | |||||||
| Performance status (ECOG) | ||||||||||||||
| 0–1 | 23 | 40 | 10 | 48 | ||||||||||
| 2 | 4 | 0 | 0.56 | 9 | 0.64 | 24 | 0.27 | |||||||
| Histology | ||||||||||||||
| SQ‡ | 22 | 14 | 9 | 38 | ||||||||||
| Adeno§ | 5 | 40 | 0.22 | 19 | 0.44 | 19 | 0.99 | |||||||
| Stage | ||||||||||||||
| III | 14 | 14 | 9 | 26 | ||||||||||
| IV | 13 | 23 | 0.65 | 17 | 0.53 | 44 | 0.21 | |||||||
| Previous response | ||||||||||||||
| Yes | 10 | 30 | 9 | 38 | ||||||||||
| No | 17 | 12 | 0.33 | 14 | 0.60 | 33 | 0.52 | |||||||
| Treatment interval (month) | ||||||||||||||
| ≤6 | 15 | 13 | 9 | 29 | ||||||||||
| >6 | 12 | 25 | 0.63 | 10 | 0.97 | 48 | 0.23 | |||||||
Time to progression.
Overall survival.
Squamous cell carcinoma.
Adenocarcinoma.
Toxicities
Hematologic and non-hematologic toxicities are listed in Table 3. CTC grade 3 granulocytopenia occurred in 1%, grade 3 thrombocytopenia in 1% and grade 3 anemia in 5% of the cycles. There were no CTC grade 4 toxicities. No patients developed febrile neutropenia. Non-hematologic toxicity was also mild.
Toxicities of second-line gemcitabine chemotherapy
| NCI CTC . | 1 . | 2 . | 3 . | 4 . | ||||
|---|---|---|---|---|---|---|---|---|
| Hematologic toxicities* (% of 87 cycles) | ||||||||
| Leukopenia | 19 (21) | 2 (2) | 0 | 0 | ||||
| Granulocytopenia | 5 (6) | 5 (6) | 1 (1) | 0 | ||||
| Thrombocytopenia | 9 (10) | 0 | 1 (1) | 0 | ||||
| Anemia | 32 (37) | 17 (19) | 4 (5) | 0 | ||||
| Non-hematologic toxicities (% of 27 patients) | ||||||||
| Nausea/Vomiting | 9 (33) | 5 (19) | 0 | 0 | ||||
| Diarrhea | 1 (4) | 0 | 0 | 0 | ||||
| Stomatitis | 4 (15) | 0 | 0 | 0 | ||||
| Alopecia | 8 (30) | 0 | 0 | 0 | ||||
| Neuropathy | 6 (22) | 0 | 0 | 0 | ||||
| Myalgia | 3 (11) | 0 | 0 | 0 | ||||
| Skin rash | 2 (7) | 0 | 0 | 0 | ||||
| NCI CTC . | 1 . | 2 . | 3 . | 4 . | ||||
|---|---|---|---|---|---|---|---|---|
| Hematologic toxicities* (% of 87 cycles) | ||||||||
| Leukopenia | 19 (21) | 2 (2) | 0 | 0 | ||||
| Granulocytopenia | 5 (6) | 5 (6) | 1 (1) | 0 | ||||
| Thrombocytopenia | 9 (10) | 0 | 1 (1) | 0 | ||||
| Anemia | 32 (37) | 17 (19) | 4 (5) | 0 | ||||
| Non-hematologic toxicities (% of 27 patients) | ||||||||
| Nausea/Vomiting | 9 (33) | 5 (19) | 0 | 0 | ||||
| Diarrhea | 1 (4) | 0 | 0 | 0 | ||||
| Stomatitis | 4 (15) | 0 | 0 | 0 | ||||
| Alopecia | 8 (30) | 0 | 0 | 0 | ||||
| Neuropathy | 6 (22) | 0 | 0 | 0 | ||||
| Myalgia | 3 (11) | 0 | 0 | 0 | ||||
| Skin rash | 2 (7) | 0 | 0 | 0 | ||||
Based on CBC done just prior to next cycle.
Toxicities of second-line gemcitabine chemotherapy
| NCI CTC . | 1 . | 2 . | 3 . | 4 . | ||||
|---|---|---|---|---|---|---|---|---|
| Hematologic toxicities* (% of 87 cycles) | ||||||||
| Leukopenia | 19 (21) | 2 (2) | 0 | 0 | ||||
| Granulocytopenia | 5 (6) | 5 (6) | 1 (1) | 0 | ||||
| Thrombocytopenia | 9 (10) | 0 | 1 (1) | 0 | ||||
| Anemia | 32 (37) | 17 (19) | 4 (5) | 0 | ||||
| Non-hematologic toxicities (% of 27 patients) | ||||||||
| Nausea/Vomiting | 9 (33) | 5 (19) | 0 | 0 | ||||
| Diarrhea | 1 (4) | 0 | 0 | 0 | ||||
| Stomatitis | 4 (15) | 0 | 0 | 0 | ||||
| Alopecia | 8 (30) | 0 | 0 | 0 | ||||
| Neuropathy | 6 (22) | 0 | 0 | 0 | ||||
| Myalgia | 3 (11) | 0 | 0 | 0 | ||||
| Skin rash | 2 (7) | 0 | 0 | 0 | ||||
| NCI CTC . | 1 . | 2 . | 3 . | 4 . | ||||
|---|---|---|---|---|---|---|---|---|
| Hematologic toxicities* (% of 87 cycles) | ||||||||
| Leukopenia | 19 (21) | 2 (2) | 0 | 0 | ||||
| Granulocytopenia | 5 (6) | 5 (6) | 1 (1) | 0 | ||||
| Thrombocytopenia | 9 (10) | 0 | 1 (1) | 0 | ||||
| Anemia | 32 (37) | 17 (19) | 4 (5) | 0 | ||||
| Non-hematologic toxicities (% of 27 patients) | ||||||||
| Nausea/Vomiting | 9 (33) | 5 (19) | 0 | 0 | ||||
| Diarrhea | 1 (4) | 0 | 0 | 0 | ||||
| Stomatitis | 4 (15) | 0 | 0 | 0 | ||||
| Alopecia | 8 (30) | 0 | 0 | 0 | ||||
| Neuropathy | 6 (22) | 0 | 0 | 0 | ||||
| Myalgia | 3 (11) | 0 | 0 | 0 | ||||
| Skin rash | 2 (7) | 0 | 0 | 0 | ||||
Based on CBC done just prior to next cycle.
Delay in the administration of gemcitabine on Day 1 was not necessary. The gemcitabine dose had to be reduced to 75% on one occasion on Days 1 and 8. The omission of gemcitabine was necessary on three (3%) occasions on Day 8 and on nine (10%) occasions on Day 15. The median administrated dose of gemcitabine on days 1, 8 and 15 was 1000 mg/m2, and the range was 750–1000, 0–1000 and 0–1000 mg/m2, respectively. The mean delivery dose of gemcitabine on Day 1, 8 and 15 was 997, 963 and 897 mg/m2, respectively. The dose intensity of gemcitabine was 714 mg/m2/week, which equates to 95% of the planned dose.
DISCUSSION
Patients in good general condition with local tumor progression or relapse of previously treated NSCLC may be candidates for second-line treatment. Of the newer cytotoxic drugs used in NSCLC, paclitaxel, vinorelbine and irinotecan as second-line treatments have yielded low response rates of 3, 0, and 9%, respectively (9–11). Docetaxel as second-line treatment in NSCLC produces a more favorable response rate of 16–21 (12,13). In a phase III study, the response rate of second-line docetaxel was lower at 7%, with a median survival time of 7 months, and 1 year survival of 29%. However, a significant improvement in the quality of life and survival was observed compared with the best supportive care alone. This suggests there is an evolving place for second-line treatment in advanced NSCLC, and docetaxel 75 mg/m2 administrated every 3 weeks may be the standard care option for these patients (14).
Second-line treatment with single-agent gemcitabine was reported to yield a tumor response rate of 6–19% with median survival of 17–28 weeks (15–17). In this phase II study of 27 uniformly pretreated patients, we obtained a response rate of 18.5% and a somewhat longer survival time (median survival, 38 weeks). These results showed that gemcitabine as a single agent is active as a second-line treatment. Our results are similar to those of Crino et al. (17), both for activity (response rate, 18.5% versus 19%) and for median survival (38 versus 34 weeks).
For patients with a relapse of small cell lung cancer, tumor response can be predicted from treatment interval, response to first-line treatment and chemotherapeutic agents used as a first-line therapy. Whether these variables also predict tumor response to second-line chemotherapy in patients with NSCLC is not known. Sculier et al. (15) have reported the effect of first-line chemotherapy and the response to gemcitabine following a response to the first-line chemotherapy in order to differentiate refractory and sensitive tumors. But this information is not fully available in the report by Crino et al. (17) and this study.
Rosvold et al. (18) have conducted a similar phase II study of 28 patients who relapsed after or were resistant to first-line carboplatin-paclitaxel treatment. Five of 24 assessable patients have sustained partial responses in addition to four minor responses. The good median survival (of ∼30 weeks) shown in this and our studies would seem to indicate that some selection of patients with favorable performance status could be an important predictive variable for response and longer survival from the second-line treatment with gemcitabine. In our study, performance status is the statistically significant factor in terms of overall survival on univariate analysis.
In the last few years, there has been a surge of interest in the number of new cytotoxic agents such as pemetrexed and targeted small molecule such as gefitinib. Recently, a large randomized phase III trial of pemetrexed versus docetaxel in patients with previously treated NSCLC has been reported. Treatment with pemetrexed resulted in clinically equivalent efficacy outcomes, but with significantly fewer side effects compared with docetaxel in the second-line treatment of patients with advanced NSCLC (19). These results suggest that pemetrexed should be considered a standard treatment option for second-line NSCLC when available.
In this second-line trial, the squamous histology is extremely high. Through a review of a previously reported trial for NSCLC at the Veterans Hospital, we can verify the predominance of squamous cell carcinoma in advanced NSCLC. Twenty (59%) patients presented with squamous cell carcinoma, eleven with adenoicarcinoma and three were unclassified NSCLC (20). In another study, the squamous histology of lung cancer in Korea is 44.7% and, among the smokers, squamous cell carcinoma (55%) is the most frequent cell type (21).
In conclusion, the reported phase II and III experiences, together with our own trial, have provided some new insights into the difficult area of second-line treatment of advanced NSCLC. Selected patients with good performance status could benefit from a second-line treatment with new active drugs. Gemcitabine seems to be promising because of its favorable toxicity profile and activity with relief of tumor-related symptoms and improved function. It is well-tolerated and can be administered on an ambulatory basis. Whether docetaxel or pemetrexed is superior to gemcitabine in second-line treatment of NSCLC needs to be further evaluated in a randomized study. We, nevertheless, need to continue searching for more effective therapies evaluated with adequate methodology to help produce.
CONCLUSION
Second-line treatment with single-agent gemcitabine showed significant activity with favorable toxicities in patients with NSCLC.
References
Central Cancer Registry Center in Korea. Ministry of Health and Welfare, Republic of Korea. Annual report of the Central Cancer Registry in Korea (1.12.2002).
Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 ramdomized clinical trials.
Bunn PA Jr, Kelly K. New chemotherapeutic agents prolong survival and improved quality of life in non-small cell lung cancer: a review of the literature and future directions.
Huisman C, Smit EF, Giaccone G, Postmus PE. Second-line chemotherapy in relapsing or refractory non-small cell lung cancer: a review.
Anderson H, Lund B, Bach F, Thatcher N, Walling J, Hansen HH. Single-agent activity on weekly gemcitabine in advanced non-small cell lung cancer: a phase II study.
Chang HJ, Ahn JB, Lee JG, Shim KY, Rha SY, Kim SK, et al. Efficacy of gemcitabine chemotherapy in advanced non-small cell lung cancer.
Bokkel Huinink WW, Bergman B, Chemaissani A. Single-agent gemcitabine: an active and better tolerated alternative to standard cisplatin-based chemotherapy in locally advanced or metastatic non-small cell lung cancer.
Bergman AM, Ruiz van Haperen V, Veerman G, Kuiper CM, Peters GJ. Synergistic interaction between cisplatin and gemcitabine in vitro.
Sculier JP, Berghmans T, Lafitte JJ, Richez M, Recloux P, Van Cutsem O, et al. A phase II study testing paclitaxel as second-line single agent treatment for patients with advanced non-small cell lung cancer failing after a first-line chemotherapy.
Pronzato P, Landucci M, Vaira F, Vigani A, Bertelli G. Failure of vinorelbine to produce responses in pretreated non-small cell lung cancer patients.
Vokes EE, Ansari RH, Masters GA, Hoffman PC, Kelpsch A, Ratain MJ, et al. A phase II study of 9-aminocamptothecin in advanced non-small cell lung cancer.
Fossella FV, Lee JS, Shin DM, Calayag M, Huber M, Perez-Soler R, et al. Phase II study of docetaxel for advanced or metastatic platinum-refractory non-small-cell lung cancer.
Gandara DR, Vokes E, Green M, Bonomi P, Devore R, Comis R, et al. Activity of docetaxel in platinum-treated non-small-cell lung cancer: results of a phase II multicenter trial.
Shepherd FA, Kancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy.
Sculier JP, Lafitte JJ, Berghmans T, Thiriaux J, Lecomte J, Efremidis A, et al. A phase II trial testing gemcitabine as second-line chemotherapy for non small cell lung cancer.
Van Putten JWG, Baas P, Codrington H, Kwa HB, Muller M, Aaronson N, et al. Activity of single-agent gemcitabine as second-line treatment after previous chemotherapy or radiotherapy in advanced non-small-cell lung cancer.
Crino L, Mosconi AM, Scagliotti G, Selvaggi G, Novello S, Rinaldi M, et al. Gemcitabine as second-line treatment for advanced non-small-cell lung cancer: a phase II trial.
Rosvold E, Langer CJ, Schilder R, Millenson M, Reimet E, Kreamer K. Salvage therapy with gemcitabine in advanced non-small cell lung cancer (NSCLC) progression after prior carboplatin-paclitaxel (C-P).
Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small cell lung cancer previously treated with chemotherapy.
Park JW, Park HY, Park YB, Kang JW, Kim HS, Lee GL, et al. A phase II study with gemcitabine and carboplatin in patients with advanced non-small cell lung cancer.