Outcomes after lung transplantation
Introduction
Although lung transplantation (LT) has become an invaluable approach for the treatment of end-stage respiratory disease, survival after the procedure is not yet as good as that after other solid-organ transplants (1). Because of this, patient survival has been the primary outcome measurement in most studies. Other indicators of outcomes like pulmonary function or quality of life have also been studied.
Survival
To date, the registry of the International Society for Heart and Lung Transplantation (ISHLT) has accrued data on more than 55,000 adult patients who received a LT in about 250 lung transplant centers from the early 90s (2). This registry provides invaluable information regarding lung transplant activity and outcome.
According to the 2016 report of this registry, adult patients who underwent primary LT between January 1990 and June 2014 had a median survival of 5.8 years, with unadjusted survival rates of 89% at 3 months, 80% at 1-year, 65% at 3 years, 54% at 5 years and 32% at 10 years (2). Post-transplant survival has improved over time with a median survival of 4.2 years in the 1990–1998 era compared to 6.1 years in the 1999–2008 era. It is remarkable that post-transplant survival continued to increase in spite of considerable change in patients’ characteristics and severity at the time of transplant. In the US for instance, between 2002 and 2014, the proportion of patients aged more than 65 years old rose from 4.5% to 28.7%, the proportion of patients being in the ICU rose from 4.2% to 15.5%, the proportion of patients under mechanical ventilation doubled, and the proportion of patients under ECMO reached 2.2% (3,4). The same shift in patient case-mix has been observed in European countries with the development of organ allocation in high emergency (5,6). Despite these improvements, survival outcomes for LT recipients remain inferior to those achieved after other solid-organ transplant procedures. For instance, the median survival after heart transplantation in the same registry is around 12 years (2).
A closer look at the LT survival curves shows that there is a large drop in early survival in the first months following LT followed by a slow attrition over time. Improvements in the management of patients in the early post-operative period led to a reduction in early mortality over the years. To this regard, some centers report on 1-year mortality well below 10% (7). However, the attrition rate after the first year, which is mainly attributable to chronic lung allograft dysfunction (CLAD) that develops in 50% of grafts at 5 years, remains largely unchanged (2).
One of the main determinants of LT outcome is the underlying disease, with a median survival of 8.9 years for cystic fibrosis (CF) patients, 6.7 years for chronic obstructive pulmonary disease (COPD) with alpha-1 antitrypsin deficiency (AATD), 5.6 years for COPD without AATD, 4.8 years for idiopathic interstitial pneumonia and 2.8 years for re-transplantation. These differences seem to be more related to differences in patients’ characteristics at the time of LT than to the underlying disease by itself. For instance, patients with COPD are older, more frequently tobacco smokers and have more comorbidities than patients with CF.
Other prognostic factors are related either to the recipient (gender, age, 6 min walking distance, patient under mechanical ventilation, dialysis or hospitalized in ICU), the donor (diabetes, age, gas exchange at the time of harvest, cause of death), the donor/recipient interaction (number of HLA mismatches, CMV or gender mismatch), the surgical approach (single vs. bilateral) and the center volume (2,8). The role of other factors like size mismatch or graft ischemic time is more debated (9-11).
Although most of these factors are not alterable, the surgical approach is. The choice between single and bilateral LT has been debated for a long time. Although the vast majority of patients with suppurative lung diseases (including CF) receive a bilateral LT (BLT), the choice of procedure remains a matter a debate for patients with COPD and IPF. Unadjusted survival rates are in favor of BLT with a median survival of 7.3 years compared to 4.6 years for single LT (SLT) recipients according to the ISHLT registry (12). However, SLT is in general proposed to older and more frail patients and analyses adjusted for patients characteristics showed conflicting results (13-15). In the absence of randomized controlled trial it is difficult to draw definitive conclusions, and the evidence comes mainly from the analysis of large registries. In COPD patients, Thabut et al. found a better survival after BLT especially in patients aged more than 60 years old (14). Schaffer et al., using more recent data but closely related methods, did not find a statistically significant difference between both surgical approaches in this indication (15). In IPF patients, although Thabut et al. failed to detect any difference in survival between both procedures (13), Schaffer et al. found better adjusted survival after BLT (15). These differences may be explained in part by differences in the characteristics of patients between the two studies, the study by Schaffer et al. including patients receiving a LT after LAS implementation.
It must be kept in mind that most of the evidence about post-transplant survival comes from large registries that lump together the outcomes of transplantations performed many years ago in centers that no longer exist with those performed in the recent years in high volume centers.
Survival benefit
Given the disappointing long-term survival of patients after LT, its ability to extend survival has been questioned (16,17). In the absence of randomized trials, appraisal of the survival benefit of LT is complex and relies on statistical modeling (18). These approaches have to deal with the following issues: patients referred to a LT center form a very selected subgroup of patients with the disease of interest, and patients who ultimately receive a LT form a selected subgroup of patients who are put on a waiting list, that may not be reflected by the characteristics of patients measured at the time of registration (18). Methods taking into account the evolution of patients’ characteristics after registration have recently been developed and provide more sensible estimates of the survival benefit of LT (18,19). Besides these technical issues, the reader must keep in mind that the results of these studies are valid for a given organ allocation system and may not apply for transplantations performed in the same indication, but in another country and may not be valid 10 years from now, because of the evolution of both pre- and post-transplant survival. In the case of CF for instance, the spontaneous life expectancy improved from 31 to 37 years over the past decade and new drugs able to dramatically change the expected survival have been developed recently (20).
About 20 studies have been published that aimed to assess the survival benefit of LT in different indications (14,16,17,19,21-34). These studies are summarized in Table 1. The survival benefit of LT is best documented in patients with IPF and CF whereas it is still debated in COPD and a lack of data precludes definitive conclusion in pulmonary arterial hypertension (PAH). In the case of COPD, a few prognostic factors have shown association with post-transplant outcome and could be used as markers to refine patient’s selection.

Full table
Quality of life
One of the main clinical aims of LT is to improve quality of life, and may be the only expected clinical benefit of LT in some indications like COPD where the survival benefit is still unclear. Quality of life encompasses many subdomains like financial status, social support, physical environment and health (36). Many studies have been published in the recent years that focused on health-related quality of life (HRQoL) before and after LT. However, the interpretation of these studies is not trivial. First, there are plenty of instruments available to measure HRQoL. Some of these instruments are generic (36-item Short Form Survey—SF-36 for instance) whereas others are disease-specific (SGRQ). In some cases, health utility measures are used that can be combined with survival to derive quality adjusted life survival. In a recent systematic review focusing on the estimation of HRQoL after LT, Seiler et al. retrieved 39 studies that used 13 different HRQoL instruments (37). Although all these instruments have advantages and limitations, they do not explore the same domains and are thus likely not to provide the same estimation of the benefit of LT. Second, a major limitation of these studies is related to the fact that patients must be well enough to fill out the questionnaires. In other words, in these studies, the data are not missing at random. For instance, in a study published 10 years ago, the authors report on the HRQoL of patients before and after LT using the SGRQ (38). In this study, patients who died after LT were excluded. This study does not allow to conclude on the improvement in HRQoL provided by LT, but only on the improvement of HRQoL in patients doing well after LT. Several methods have been used to account for these missing not at random data, like imputing the worst possible HRQoL to those who died post-transplant or combining survival and HRQoL (39).
All the studies focusing on HRQoL after LT found dramatic improvements in HRQoL regardless of the indication for LT and whether HRQL is measured by generic, respiratory-specific HRQL instruments, or by utility measures (36,37). Table 2 reports the benefit of LT on quality of life measured by both generic and specific tools.
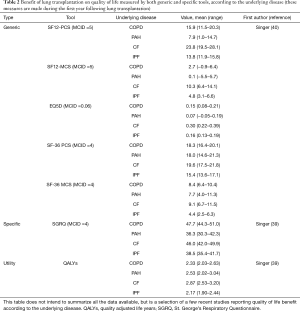
Full table
The most popular generic HRQoL instrument is the SF-36. The SF-36 features physical and mental summary scores (PCS and MCS), and a 4-point change in the SF-36 is considered clinically significant (MCID). In a multicenter randomized controlled trial about CMV prophylaxis, SF-36 was measured before transplantation and every 3 months up to 1 year after LT (41). The authors observed a 10.9 points improvement in PCS score, almost reaching the norms of the US population. Concomitant with increased PCS scores, they also found increase in the subdomains that contribute to PCS: physical function, role-physical, and general health. In contrast, the MCS did not change from baseline level, remaining well below the US population norm throughout the first post-operative year. Further evaluation of the MCS domains showed that mental health and vitality domain scores did not improve, whereas increases were observed in social function and role-emotional domains. In a recent prospective study involving 326 patients that contributed to HRQoL measurements both before and after LT, a 17.7 improvement in the SF-36 physical component score was observed (39). Again, the improvement in the mental component score was more modest (7.8 points). Other studies using the SF-36 or other HRQoL instruments have reported mostly the same results. Similar results were found in studies performed after the introduction of the LAS score in the US (40).
The same results have been found in studies using respiratory-specific HRQoL. One of the most popular disease-specific HRQoL tool is the St. George’s Respiratory Questionnaire (SGRQ) that provides a summary score and a score for 3 sub-domains: impact, symptoms and activity. In a prospective cohort study involving 326 patients in whom HRQoL has been measured pre and post transplantation using various questionnaires, average improvements in SGRQ was 47 points, which is more than 10 times the MCID for this tool (39). These changes greatly exceed those seen with other treatments for advances lung disease. For instance, in recent studies on bronchoscopic lung volume reduction (BLVR) in COPD patients, the mean improvements in total SGRQ was 13.4 points (42) to be compared to 49.9 points improvements in patients receiving a LT in the study by Singer et al. (39). In another study, an improvement of 33 points was found, with similar improvements in the 3 domain scores (43). This improvement persisted even when the worst possible values were imputed to patients who died after LT. These improvements were similar to those found after other solid organ transplantations (44).
Most studies focused on the first years following LT and very few studies reported on QoL of patients surviving more than 3 years after LT. As such, the trajectory of QoL beyond 3 years post-transplant remains uncertain. A few factors have been associated with post-transplant QoL. CLAD developing in about 50% of patients at 5 years appears to be the strongest determinant of physical health status (45). The other predictors of HRQoL after LT were immunosuppressants side-effects, indication for LT, older age at the time of transplant, a single-lung transplant, and recurrent infections (37).
Pulmonary function tests
The pulmonary function of transplant recipients results from pre-transplant factors (underlying disease in the case of SLT), operative factors (pleural or diaphragmatic injury) and post-transplant complications (bronchial strictures). In the first weeks after LT, pulmonary function is hampered by various factors including pain and early graft dysfunction, and the peak in pulmonary function is in general observed between 3 to 12 months following LT. The average function declines thereafter because of CLAD that develops in 50% of patients at 5 years. Surgical approach (SLT vs. BLT) and underlying disease in case of SLT are the two main factors associated with post-transplant pulmonary function.
Patients who receive a BLT typically achieve normal pulmonary function tests (FEV1, FVC, TLC) as well a gas exchange whatever the indication for LT (46). Lower PFTs are achieved following SLT and depend on the indication. Almost normal FEV1 can be expected in patients with PAH, whereas IPF patients have typically FEV1 between 60 and 80 percent of predicted value and COPD patients achieve typically FEV1 in the 50–60% range (47). Blood gases are typically normal. Small sample size studies performed many years ago have shown that considerable exercise limitations persisted after either single or bilateral LT despite pulmonary function restoration, with VO2 around 50% of predicted values (48). Similar results were found in a study including 153 patients in recent years (49,50). Interestingly, BLT did not result in better exercise tolerance than SLT (49,50). The skeletal muscle appears to be the cause of exercise limitation in most and may, in part, reflect persistence of a pre-transplant skeletal muscle injury (46).
Other outcomes have been reported like employment status. For instance, in the ISHLT registry, at 5 years post-transplant, about 40% of patients are not working, 30% are retired and a little less than 20% are working part or full-time. However, these figures are likely to vary from country to country and pose the same issues of missing values at that already mentioned for HRQoL.
In conclusion, LT allows for major improvements in lung function and exercise tolerance that translates into dramatic improvement in HRQoL that far exceeds the effects of other treatments of end-stage lung diseases. Although recent studies suggest that LT improves survival in most cases, post-transplantation survival remains hampered by the frequent development of CLAD. A better understanding of the mechanisms implicated in CLAD development could allow to match the outcomes after other solid organ transplantations.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kotloff RM, Thabut G. Lung transplantation. Am J Respir Crit Care Med 2011;184:159-71. [Crossref] [PubMed]
- Yusen RD, Edwards LB, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Lung and Heart-Lung Transplant Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant 2016;35:1170-84. [Crossref] [PubMed]
- Valapour M, Skeans MA, Smith JM, et al. Lung. Am J Transplant 2016;16 Suppl 2:141-68. [Crossref] [PubMed]
- Valapour M, Skeans MA, Heubner BM, et al. OPTN/SRTR 2012 Annual Data Report: lung. Am J Transplant 2014;14 Suppl 1:139-65. [Crossref] [PubMed]
- Lafarge M, Mordant P, Thabut G, et al. Experience of extracorporeal membrane oxygenation as a bridge to lung transplantation in France. J Heart Lung Transplant 2013;32:905-13. [Crossref] [PubMed]
- Boussaud V, Mal H, Trinquart L, et al. One-year experience with high-emergency lung transplantation in France. Transplantation 2012;93:1058-63. [Crossref] [PubMed]
- Transplant Center Search Results. Accessed on June 1, 2017. Available online: https://www.srtr.org/transplant-centers/?&organ=lung&recipientType=adult&sort=volume
- Thabut G, Christie JD, Kremers WK, et al. Survival differences following lung transplantation among US transplant centers. Jama 2010;304:53-60. [Crossref] [PubMed]
- Thabut G, Mal H, Cerrina J, et al. Graft ischemic time and outcome of lung transplantation: a multicenter analysis. Am J Respir Crit Care Med 2005;171:786-91. [Crossref] [PubMed]
- Eberlein M, Reed RM, Maidaa M, et al. Donor-recipient size matching and survival after lung transplantation. A cohort study. Ann Am Thorac Soc 2013;10:418-25. [Crossref] [PubMed]
- Eberlein M, Reed RM, Bolukbas S, et al. Lung size mismatch and primary graft dysfunction after bilateral lung transplantation. J Heart Lung Transplant 2015;34:233-40. [Crossref] [PubMed]
- Registry I. 2016 Slides. Accessed 06/03/2017. Available online: https://www.ishlt.org/registries/slides.asp?slides=heartLungRegistry
- Thabut G, Christie JD, Ravaud P, et al. Survival after bilateral versus single-lung transplantation for idiopathic pulmonary fibrosis. Ann Intern Med 2009;151:767-74. [Crossref] [PubMed]
- Thabut G, Christie JD, Ravaud P, et al. Survival after bilateral versus single lung transplantation for patients with chronic obstructive pulmonary disease: a retrospective analysis of registry data. Lancet 2008;371:744-51. [Crossref] [PubMed]
- Schaffer JM, Singh SK, Reitz BA, et al. Single- vs double-lung transplantation in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis since the implementation of lung allocation based on medical need. JAMA 2015;313:936-48. [Crossref] [PubMed]
- Liou TG, Adler FR, Cox DR, et al. Lung transplantation and survival in children with cystic fibrosis. N Engl J Med 2007;357:2143-52. [Crossref] [PubMed]
- Hosenpud JD, Bennett LE, Keck BM, et al. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet 1998;351:24-7. [Crossref] [PubMed]
- Thabut G. Estimating the Survival Benefit of Lung Transplantation: Considering the Disease Course during the Wait. Ann Am Thorac Soc 2017;14:163-4. [PubMed]
- Vock DM, Durheim MT, Tsuang WM, et al. Survival Benefit of Lung Transplantation in the Modern Era of Lung Allocation. Ann Am Thorac Soc 2017;14:172-81. [PubMed]
- Accurso FJ, Rowe SM, Clancy JP, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med 2010;363:1991-2003. [Crossref] [PubMed]
- Titman A, Rogers CA, Bonser RS, et al. Disease-specific survival benefit of lung transplantation in adults: a national cohort study. Am J Transplant 2009;9:1640-9. [Crossref] [PubMed]
- Thabut G, Mal H, Castier Y, et al. Survival benefit of lung transplantation for patients with idiopathic pulmonary fibrosis. J Thorac Cardiovasc Surg 2003;126:469-75. [Crossref] [PubMed]
- Thabut G, Christie JD, Mal H, et al. Survival benefit of lung transplant for cystic fibrosis since lung allocation score implementation. Am J Respir Crit Care Med 2013;187:1335-40. [Crossref] [PubMed]
- Tanash HA, Riise GC, Hansson L, et al. Survival benefit of lung transplantation in individuals with severe alpha1-anti-trypsin deficiency (PiZZ) and emphysema. J Heart Lung Transplant 2011;30:1342-7. [Crossref] [PubMed]
- Stavem K, Bjortuft O, Borgan O, et al. Lung transplantation in patients with chronic obstructive pulmonary disease in a national cohort is without obvious survival benefit. J Heart Lung Transplant 2006;25:75-84. [Crossref] [PubMed]
- Russo MJ, Worku B, Iribarne A, et al. Does lung allocation score maximize survival benefit from lung transplantation? J Thorac Cardiovasc Surg 2011;141:1270-7. [Crossref] [PubMed]
- Liou TG, Adler FR, Huang D. Use of lung transplantation survival models to refine patient selection in cystic fibrosis. Am J Respir Crit Care Med 2005;171:1053-9. [Crossref] [PubMed]
- Liou TG, Adler FR, Cahill BC, et al. Survival effect of lung transplantation among patients with cystic fibrosis. Jama 2001;286:2683-9. [Crossref] [PubMed]
- Lahzami S, Bridevaux PO, Soccal PM, et al. Survival impact of lung transplantation for COPD. Eur Respir J 2010;36:74-80. [Crossref] [PubMed]
- Hofer M, Benden C, Inci I, et al. True survival benefit of lung transplantation for cystic fibrosis patients: the Zurich experience. J Heart Lung Transplant 2009;28:334-9. [Crossref] [PubMed]
- Geertsma A, Ten Vergert EM, Bonsel GJ, et al. Does lung transplantation prolong life? A comparison of survival with and without transplantation. J Heart Lung Transplant 1998;17:511-6. [PubMed]
- De Meester J, Smits JM, Persijn GG, et al. Listing for lung transplantation: life expectancy and transplant effect, stratified by type of end-stage lung disease, the Eurotransplant experience. J Heart Lung Transplant 2001;20:518-24. [Crossref] [PubMed]
- Charman SC, Sharples LD, McNeil KD, et al. Assessment of survival benefit after lung transplantation by patient diagnosis. J Heart Lung Transplant 2002;21:226-32. [Crossref] [PubMed]
- Aurora P, Whitehead B, Wade A, et al. Lung transplantation and life extension in children with cystic fibrosis. Lancet 1999;354:1591-3. [Crossref] [PubMed]
- Thabut G, Ravaud P, Christie JD, et al. Determinants of the survival benefit of lung transplantation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:1156-63. [Crossref] [PubMed]
- Singer JP, Singer LG. Quality of life in lung transplantation. Semin Respir Crit Care Med 2013;34:421-30. [Crossref] [PubMed]
- Seiler A, Klaghofer R, Ture M, et al. A systematic review of health-related quality of life and psychological outcomes after lung transplantation. J Heart Lung Transplant 2016;35:195-202. [Crossref] [PubMed]
- Gerbase MW, Spiliopoulos A, Rochat T, et al. Health-related quality of life following single or bilateral lung transplantation: a 7-year comparison to functional outcome. Chest 2005;128:1371-8. [Crossref] [PubMed]
- Singer LG, Chowdhury NA, Faughnan ME, et al. Effects of Recipient Age and Diagnosis on Health-related Quality-of-Life Benefit of Lung Transplantation. Am J Respir Crit Care Med 2015;192:965-73. [Crossref] [PubMed]
- Singer JP, Katz PP, Soong A, et al. Effect of Lung Transplantation on Health-Related Quality of Life in the Era of the Lung Allocation Score: A U.S. Prospective Cohort Study. Am J Transplant 2017;17:1334-45. [Crossref] [PubMed]
- Finlen Copeland CA, Vock DM, Pieper K, et al. Impact of lung transplantation on recipient quality of life: a serial, prospective, multicenter analysis through the first posttransplant year. Chest 2013;143:744-50. [Crossref] [PubMed]
- Deslée G, Mal H, Dutau H, et al. Lung Volume Reduction Coil Treatment vs Usual Care in Patients With Severe Emphysema: The REVOLENS Randomized Clinical Trial. Jama 2016;315:175-84. [Crossref] [PubMed]
- Eskander A, Waddell TK, Faughnan ME, et al. BODE index and quality of life in advanced chronic obstructive pulmonary disease before and after lung transplantation. J Heart Lung Transplant 2011;30:1334-41. [Crossref] [PubMed]
- Kugler C, Gottlieb J, Warnecke G, et al. Health-related quality of life after solid organ transplantation: a prospective, multiorgan cohort study. Transplantation 2013;96:316-23. [Crossref] [PubMed]
- Künsebeck HW, Kugler C, Fischer S, et al. Quality of life and bronchiolitis obliterans syndrome in patients after lung transplantation. Prog Transplant 2007;17:136-41. [Crossref] [PubMed]
- Williams TJ, Grossman RF, Maurer JR. Long-term functional follow-up of lung transplant recipients. Clin Chest Med 1990;11:347-58. [PubMed]
- Mal H, Sleiman C, Jebrak G, et al. Functional results of single-lung transplantation for chronic obstructive lung disease. Am J Respir Crit Care Med 1994;149:1476-81. [Crossref] [PubMed]
- Williams TJ, Patterson GA, McClean PA, et al. Maximal exercise testing in single and double lung transplant recipients. Am Rev Respir Dis 1992;145:101-5. [Crossref] [PubMed]
- Orens JB, Becker FS, Lynch JP 3rd, et al. Cardiopulmonary exercise testing following allogeneic lung transplantation for different underlying disease states. Chest 1995;107:144-9. [Crossref] [PubMed]
- Bartels MN, Armstrong HF, Gerardo RE, et al. Evaluation of pulmonary function and exercise performance by cardiopulmonary exercise testing before and after lung transplantation. Chest 2011;140:1604-11. [Crossref] [PubMed]


