Article Text
Abstract
Objective To compare the efficacy of inhaled corticosteroids (ICS) versus montelukast (MONT) in schoolchildren and adolescents with mild–moderate persistent asthma.
Methods Randomised, prospective, controlled trials published January 1996 to November 2009 with a minimum of 4 weeks of ICS versus MONT and of ICS versus MONT+ICS were retrieved through Medline, Embase and Central databases. The primary outcome was asthma exacerbations requiring systemic corticosteroids (AEX); secondary outcomes were pulmonary function, withdrawal/hospitalisation due to AEX, change in symptoms score, rescue-medication-free days, albuterol use, adverse effects and adherence.
Results Of 124 studies identified, 18 studies (n=3757 patients) met criteria for inclusion (13 compared ICS vs MONT, 3 ICS vs MONT+ICS and 2 ICS vs MONT vs ICS+MONT). Patients receiving ICS showed a significantly lower risk for AEX than those with MONT (RR=0.83, 95% CI 0.72 to 0.96, p=0.01); post-hoc analysis suggests this effect was independent of quality, sponsorship and study duration. Children treated with ICS had a significant higher pulmonary function (final FEV1 % predicted, change from baseline FEV1 %, final morning peak expiratory flow (PEF)) and better clinical parameters (albuterol use, symptom score, rescue-medication-free days, withdrawals due to AEX) versus MONT. No significant difference in primary or secondary outcomes was found when MONT was added on to ICS versus ICS alone; however, these analyses were based on only two studies.
Conclusions Schoolchildren and adolescents with mild-moderate persistent asthma treated with ICS had less AEX and better lung function and asthma control than with MONT. There are insufficient data to determine whether the addition of MONT to ICS improves outcome.
Statistics from Altmetric.com
Introduction
Asthma is one of the most common chronic diseases in children worldwide.1 All current international guidelines recommend the use of low-dose (200–400 µg of beclomethasone (BDP) or equivalent) inhaled corticosteroids (ICS) as the preferred controller therapy, with leucotriene receptor antagonist (LTRA) as an alternative, for the management of persistent asthma in children (5–11 years of age) and adolescents. In patients unresponsive to ICS alone, options include the addition of LTRA or long-acting β-agonist (LABA), or an increase the dose of ICS.2,–,4
What is already known on this topic
In children with mild–moderate asthma, two main controllers have been used: inhaled corticosteroids and montelukast.
What this study adds
This extensive meta-analysis shows that in schoolchildren and adolescents (n=3757) with mild–moderated persistent asthma, inhaled corticosteroids were significantly better than montelukast in preventing severe asthma exacerbation (requiring systemic corticosteroids) and in improving lung function and asthma control.
When evaluating therapies in an evidence base fashion, a meta-analysis that includes a substantial number of randomised controlled trials (RCTs) is generally considered a higher level of evidence than smaller, individual studies. More than 5 years ago, two meta-analyses comparing ICS and LTRA were reported.5 6 One demonstrated that ICS (400 µg/day of BDP or equivalent) is more effective than LTRA in reducing the number of exacerbations requiring systemic corticosteroids (the primary outcome).5 However, the only three paediatric trials published to that point could not be included because two reported no exacerbation events. The authors noted the need for more studies in children.5 The second meta-analysis showed that LTRA added on to ICS brings only a modest improvement in lung function, but not a significant reduction in exacerbations requiring systemic steroids (the main outcome) compared with ICS alone. However, only one trial in children was included.6 Since then, more randomised trials comparing ICS and LTRA in children have been conducted; therefore, it seems reasonable to explore this new evidence.
Thus, the objective of this systematic review is to compare the efficacy of ICS versus montelukast (MONT) (the most common LTRA use in children worldwide) and versus MONT added on to ICS in schoolchildren and adolescents with persistent asthma.
Methods
Search strategy and eligibility criteria
We identified studies from Medline (January 1966 to November 2009), Embase (January 1980 to November 2009) and the Cochrane Controlled Trials Register (central) (third quarter 2009) databases by using the following Medical Subject Headings (MeSH), full-text and keyword terms: montelukast or antileucotriene and steroid, corticosteroid or beclomethasone or budesonide or fluticasone or triamcinolone or flunisolide.
Inclusion criteria for trials included (1) children aged less than 18 years with a clinical diagnosis of asthma for at least 6 months before study entry; (2) RCTs (parallel group or crossover) without language restriction; (3) a minimum of 4 weeks of treatment with ICS compared with MONT or with ICS plus MONT (the dose of ICS was maintained throughout the intervention period); (4) primary outcome measure of asthma exacerbations (AEX), defined as worsening symptoms that required systemic corticosteroid use; and (5) secondary outcome measures of final pulmonary function (forced expiratory volume in the first second (FEV1)), mean change from baseline in pulmonary function (FEV1), final morning peak expiratory flow (PEF), mean change from baseline in albuterol use, mean change in symptom score, mean rescue-medication-free days, hospitalisations due to AEX, final eosinophil count, all-cause withdrawals, withdrawals due to AEX, incidence of overall adverse effects, and adherence to treatment. Trials published solely in abstract form were excluded because methods and results could not be fully analysed.
Data abstraction and quality assessment
Titles, abstracts and citations were reviewed independently by two reviewers (Drs Castro-Rodriguez and Rodrigo) to assess potential relevance for full review. From the full text, both reviewers independently assessed candidate studies for inclusion on the basis of the criteria for population, intervention, study design and outcomes. Data extraction included: (1) age, gender, number of patients studied, patient demographics and withdrawals; (2) agent, dose, route of delivery and duration of therapy; (3) concurrent control treatments; (4) outcomes; (5) method of randomisation and allocation concealment; and (6) sponsorship of the study. The methodological quality of each candidate trial was evaluated by using the five-point scale (0=worst, 5=best) described by Jadad et al.7 This instrument assesses the adequacy of randomisation and blinding, as well as the handling of withdrawals and dropouts.
Statistical analysis
Binary outcomes were pooled by using common relative risks (RRs) and 95% CI. If pooled effect estimates for dichotomous outcomes were significantly different between groups, we calculated the number needed to treat (NNT). For continuous outcomes, the standardised mean difference (SMD) (for variables using different units of measure) and weighted mean difference (WMD) (for variables using the same unit) and 95% CIs were calculated. We interpreted the effect size using the guidelines of Cohen8 that a small effect size is 0.2 SD units, a medium effect size is 0.5 SD units, and a large effect size is ≥0.8 SD. Heterogeneity was further measured using the I2 test.9 With low heterogeneity (I2 < 40%), data were combined by means of a fixed-effects model;9 10 otherwise, a random-effects model was used.11 A predefined sensitivity analysis of the primary outcome was conducted to explore the influence of the following factors on the results: duration of treatment (>24 weeks vs ≤ 24 weeks); quality assessment (Jadad score ≥4 vs <4); and sponsorship of the study (pharmaceutical companies vs independent). Subgroups were compared using the interaction test.12 A p value of 0.05 using a two-tailed test was considered to indicate significance. The fail-safe number, using the Rosenberg method13 was calculated to assess the potential impact of unpublished studies on the analysis. This number indicates the number of non-significant unpublished studies that would need to be added to a meta-analysis to reverse an overall statistically significant result to non-significance. A fail-safe number is often considered robust if it is greater than 5n+10, where n is the original number of studies.13 Meta-analyses were performed with the Review Manager 5.0.18 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2009), and intention-to-treat analyses were used when possible.
Results
A total of 124 abstracts were identified in the initial search. Of these, 18 RCTs including 3757 patients met the inclusion criteria and were selected for analysis.14,–,31 Details of the selection process are shown in figure 1. There was total agreement between the reviewers on inclusion of studies. Although two studies were based on the same data set, both were included in this analysis because they reported different outcomes, FEV121 and symptoms score.23 Thirteen studies compared ICS vs MONT,14,–,26 three studies compared ICS vs MONT plus ICS,27,–,29 and two studies tested three protocols (ICS vs MONT vs ICS plus MONT)30 31 (table 1). Seven studies included budesonide,20 24 27,–,31 four BDP,14 15 22 26 five fluticasone propionate18 19 21 23,–,25 and two triamcinolone.16 17 The mean age of patients was 9.7 years (63% of males) with an average baseline FEV1 of 81% of predicted normal values. Only one study included children aged less than 8 years (2–8 years);26 in that study ICS were administered via a nebuliser. All the remaining trials included asthmatics from 5–18 years of age and used metered dose inhaler or dry powder devices to administered ICS. There were six long-term trials (>24 weeks), and eight were of high methodological quality (Jadad≥4). No trials reported the use of additional antiasthmatic drugs other than rescue β2-agonists and oral corticosteroids.
Flow chart for identification of usable studies. ICS, inhaled corticosteroids.
Characteristics of selected studies (n=3757)
Asthma exacerbations
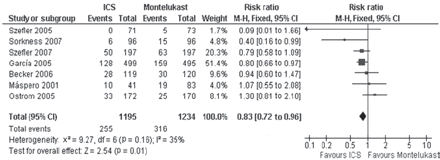
Seven studies (2429 asthmatics)14 18 19 21 22 25 26 comparing ICS with MONT presented data on AEX requiring systemic corticosteroids; patients treated with ICS showed a significant decrease in risk of an AEX as compared with those treated with MONT (RR=0.83, 95% CI 0.72 to 0.96, I2=35%, p=0.01) (figure 2). The overall cumulative incidence was 21.3% in the ICS group and 25.6% in the MONT group, with a risk difference of 4.3% (95% CI 0.9% to 7.6%). This reduction can be reported as 43 patients out of 1000 benefiting from ICS therapy (95% CI 9 to 76). The NNT with ICS instead MONT to prevent one extra exacerbation was 24 (95% CI 13 to 110). The fail-safe number calculated was 133 (number of studies required to reverse the significant decreased risk of AEX seen with ICS). The post-hoc subgroup analysis (table 2) showed that factors such as duration of treatment, quality of trials and sponsorship of the study did not influence the effect size of AEX. Because only one study included children between 2 and 8 years of age,26 we excluded it in a new analysis limited to the remaining trials (range 5–18 years of age). However, this exclusion did not change the primary conclusions on the incidence of AEX (RR=0.84; 95% CI 0.72 to 0.99, I2=38%, p=0.03).
Pooled relative risk for asthma exacerbations requiring systemic corticosteroids (with 95% CI) of eligible studies comparing inhaled corticosteroids (ICS) vs montelukast.
Sensitivity analysis of asthma exacerbations (risk in children on inhaled corticosteroids as compared with children on Montelukast)
In contrast, there was no significant difference in the incidence of patients experiencing AEX between ICS vs ICS plus MONT groups (RR=0.53, 95% CI 0.10 to 2.74, I2=86%, p=0.45). However, this analysis was based on only two studies27 28 and presented evidence of statistical heterogeneity.
Secondary outcomes
Twelve studies14,–,20 24,–,26 30 31 showed that the final pulmonary function (FEV1 % predicted) of patients who received ICS was significantly higher than in those patients treated with MONT (table 3). In the same way, children treated with ICS showed a significantly higher mean change from baseline in pulmonary function (FEV1), final morning PEF and mean rescue-medication-free days, and both significantly lower albuterol use and incidence of all-cause withdrawals, compared with those children that received MONT. On the other hand, there were no significant differences in the incidence of overall adverse effects, hospitalisations due to AEX, final eosinophil count, withdrawals due to AEX and adherence to treatment between both groups.
Analysis of secondary outcomes (ICS vs Montelukast)
Finally, the addition of MONT to ICS was equivalent to ICS alone in all secondary outcomes (table 4). Nevertheless, it must be emphasised that these results were based on a small number of trials.
Analysis of secondary outcomes (ICS vs ICS+Montelukast)
Discussion
To our knowledge, this is the most extensive meta-analysis performed exclusively to explore the efficacy of ICS compared with MONT and ICS compared with MONT plus ICS in schoolchildren and adolescents with mild to moderate persistent asthma. Overall, ICS therapy was associated with a significant reduction in the incidence of AEX requiring systemic corticosteroids compared with MONT. This beneficial effect was independent of quality, duration and sponsorship of the study. Similarly, there was no significant difference in AEX when MONT was added on to ICS vs ICS alone; however this result was based on only two studies.
The superiority of ICS versus MONT shown in the present meta-analysis agrees with a recent review on five paediatric trials reporting that patients with ICS had better pulmonary function and higher asthma control days than those with MONT;32 and with the recent international guidelines, which stated that ICS is the cornerstone of treatment at step 2 or higher for asthmatic children.2,–,4 The latest version of the National Asthma Education and Prevention Program guidelines3 distinguishes two dimensions in the assessment of asthma control: impairment (that includes frequency of asthma symptoms, nocturnal awakenings, use of quick-relief medications and level of lung function) and risk (the most important component of which is the likelihood of asthma exacerbations).
In the present meta-analysis, we found that children who received ICS showed a significant decrease in risk for an AEX than those treated with MONT. This is a very relevant finding, since preventing exacerbations remains the most important challenge in asthma treatment,33 has the greatest impact on healthcare utilisation and treatment costs for children with asthma,34 and may reduce the most important cause of loss of school days for children with asthma.35 The NNT of 24 children with ICS for avoiding an extra AEX reported here is, of course, higher than the NNT of seven comparing ICS versus placebo in preschoolers with recurrent wheezing or asthma,36 but is very similar to the NNT of 22 comparing LABA plus ICS versus ICS.37 Moreover, the advantage of ICS over MONT in reducing AEX found here was observed in both short-term and long-term trials (>24 weeks). The goal of asthma therapy is to maintain the longest duration possible in controlling asthma with the least amount of medication and, hence, with the lowest risk for adverse effects.2,–,4 In the past it was speculated that the adherence to oral MONT therapy would be higher than that to ICS. However, in the present study, the adherence to MONT and ICS was similar, confirming a previous result of a real-world study.38
Regarding secondary outcomes, schoolchildren and adolescents treated with ICS had a significantly higher final pulmonary function (final FEV1 % predicted, change from baseline FEV1 % and final morning PEF) and better clinical outcomes of asthma control (albuterol use, symptom score, rescue-medication-free days and withdrawals due to AEX) than those treated with MONT. Although these differences were statistically significant, the clinical relevance of these improvements remains elusive (most of them show a small effect size (SMD < 0.2 SD units)) and needs further study.
According to the international guidelines, in children and adolescents with persistent asthma that do not achieve control of their disease with low doses of ICS, an LTRA or LABA should be added.2,–,4 However, this recommendation comes from studies done in adults and adolescents greater than 15 years old. A previous Cochrane meta-analysis (in which only one paediatric study was included) showed only a modest effect of adding LTRA on morning PEF rate, β2 agonists and eosinophil counts, but not in the main outcome (AEX required systemic corticosteroids) nor on change in FEV1 or other clinical outcomes (ie, symptom score, nocturnal awakenings or quality of life) in asthmatics.6 In the present study, we found no significant difference in the primary (AEX) or secondary (pulmonary function, change on symptom score, β2 agonist use, withdrawals due to AEX or final eosinophil count) outcomes when MONT was added to ICS versus ICS alone in children (5–18 years of age) with persistent asthma. Nevertheless, these results were based on only two studies which had evidence of statistical heterogeneity. However, if more studies in children confirm these results, modification of the guidelines regarding the addition of LTRA should be considered.
This study met most of the methodological criteria suggested for scientific reviews.39 All included studies were randomised and combined with quite homogeneous clinical characteristics of the studied samples (namely asthma severity). The high fail-safe number calculated gives robust evidence against publication bias. Thus, it is unlikely that 123 studies (the number of studies that would be required to reverse our conclusions regarding AEX) would be found with a more extensive literature search. An important limitation was that the analysis of the main outcome was based on only seven studies (representing 65% of total sample). Also, stratification of studies according to different relevant factors was not always possible. It is important to underline that in terms of our primary outcome, there was a trend (p=0.07) in which independent RCT studies found a greater beneficial effect of ICS versus MONT in comparison with the RCT studies sponsored by pharmaceutical companies. Although we found a similar incidence of overall adverse effects and withdrawals between ICS and MONT, the adverse effects typically associated with ICS, such as growth suppression and, less frequently, osteopenia and adrenal suppression, have not been measured in these trials.
In conclusion, this meta-analysis shows that in school-age children and adolescents with mild to moderate persistent asthma, ICS (200–300 µg/day of BDP or equivalent) was superior to MONT (5–10 mg/day) in preventing AEX requiring systemic corticosteroids (an effect observed in a long-term trials), and improving lung function and asthma control (ie, albuterol use, symptoms score, medication-free days and incidence of all-cause withdrawals).The addition of MONT to ICS (200–400 µg/day of BDP or equivalent) did not improve the primary and secondary outcomes compared with treatment with ICS alone; however, since this analysis was based on only two trials, more studies need to be done to clarify this point.
Acknowledgments
We thank MA Brown (from the Pediatric Pulmonary Section, University of Arizona) for his advice and critical review of the manuscript.
References
Footnotes
-
Competing interests JAC-R has received lecturing and consultancy fees from Merck Sharp & Dohme, GlaxoSmithKline and Grünenthal. GJR has participated as a lecturer and speaker in scientific meetings and courses under the sponsorship of Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, Dr Esteve SA and Merck Sharp & Dome. He also received honoraria as a consultant for CYDEX and Discovery Laboratories. No sponsorship from institutions or pharmaceutical industry was provided to conduct this study. No pharmaceutical company sponsored this study or had any role in the study design, data collection, data analysis, data interpretation or writing of the manuscript.
-
Provenance and peer review Not commissioned; externally peer reviewed.
-
Patient consent Obtained.