Article Text
Abstract
Background Exercise-induced respiratory symptoms are common among adolescents. Exercise is a known stimulus for transient narrowing of the airways, such as exercise-induced bronchoconstriction (EIB) and exercise-induced laryngeal obstruction (EILO). Our aim was to investigate the prevalence of EIB and EILO in a general population of adolescents.
Methods In this cross-sectional study, a questionnaire on exercise-induced dyspnoea was sent to all adolescents born in 1997 and 1998 in Uppsala, Sweden (n=3838). A random subsample of 146 adolescents (99 with self-reported exercise-induced dyspnoea and 47 without this condition) underwent standardised treadmill exercise tests for EIB and EILO. The exercise test for EIB was performed while breathing dry air; a positive test was defined as a decrease of ≥10% in FEV1 from baseline. EILO was investigated using continuous laryngoscopy during exercise.
Results The estimated prevalence of EIB and EILO in the total population was 19.2% and 5.7%, respectively. No gender differences were found. In adolescents with exercise-induced dyspnoea, 39.8% had EIB, 6% had EILO and 4.8% had both conditions. In this group, significantly more boys than girls had neither EIB nor EILO (64.7% vs 38.8%; p=0.026). There were no significant differences in body mass index, lung function, diagnosed asthma or medication between the participants with exercise-induced dyspnoea who had or did not have a positive EIB or EILO test result.
Conclusions Both EIB and EILO are common causes of exercise-induced dyspnoea in adolescents. EILO is equally common among girls and boys and can coexist with EIB.
Statistics from Altmetric.com
Key messages
What is the key question?
-
What is the prevalence of exercise-induced bronchoconstriction (EIB) and exercise-induced laryngeal obstruction (EILO) in a general adolescent population assessed using standardised exercise tests?
What is the bottom line?
-
The prevalence of EIB is 19.2% and the prevalence of EILO is 5.7% with no gender differences.
Why read on?
-
This study shows that a large proportion of adolescents have EIB and EILO assessed using standardised methodology.
Introduction
Exercise-induced respiratory symptoms are common among adolescents. Exercise-induced dyspnoea was reported by 14% of Swedish adolescents1 and exercise-related wheezing was reported by 19% in the International Study of Asthma and Allergies.2 Although common, the reasons for exercise-induced respiratory symptoms have not been thoroughly investigated and are not well understood. Two possible causes of respiratory problems in conjunction with exercise are exercise-induced bronchoconstriction (EIB)3 ,4 and exercise-induced laryngeal obstruction (EILO).3 ,5 ,6
Several studies have investigated the prevalence of EIB, a transient narrowing of the lower airways provoked by exercise. The prevalence in general populations of older children and adolescents varies between 7% and 16%7–11 depending on the population under study, the diagnostic criteria and the environmental conditions. Although the studies used exercise tests for investigating EIB, not all of the studies used standardised tests and none of them used inhalation of dry air during the test, as recommended in the American Thoracic Society (ATS) clinical practice guidelines.4
EILO comprises a group of conditions that involves the vocal cords and the supraglottic structures in the larynx where the airflow in the larynx is obstructed during physical exertion. EILO is diagnosed by continuous laryngoscopy during exercise.5 ,12 ,13 The only prospective study that has investigated the prevalence of EILO reported that it occurred in 7.5% of a cohort of subjects aged 14–24 years.14
Despite their different pathophysiological backgrounds, EIB and EILO can have similar symptomatology. For the correct diagnosis and treatment of adolescents with exercise-induced respiratory symptoms, it would be useful if clinicians were aware of the prevalence of EIB and EILO, whether there are any gender differences and the differences in clinical characteristics between those with and without these conditions. Previous studies investigating EIB with exercise tests were not performed according to recent guidelines, and only one prospective study has investigated the prevalence of EILO in a general population. Therefore, the aim of this study was to explore and assess the prevalence of EIB and EILO using standardised exercise tests in a general population of adolescents.
Methods
Participants and study design
This cross-sectional study comprised two phases over a 2-year period: a screening phase and a clinical phase (figure 1).
Participants’ inclusion and exclusion. EIB, exercise-induced bronchoconstriction; CLE, continuous laryngoscopy exercise test.
Screening phase
During 2011 all 12–13-year-old adolescents in the city of Uppsala (n=3838) received a questionnaire on exercise-induced respiratory problems.1 The adolescents were also asked to report exercise-induced respiratory symptoms by answering the following question: ‘Have you had any of the following symptoms more than once during or after physical exercise during the last 12 months; wheeze, chest tightness, cough, throat tightness, choking sensation, hoarseness or inspiratory stridor?’.
The response rate was 60.2%. Based on the answer to the question ‘Have you had an attack of shortness of breath that came following strenuous activity at any time, in the last 12 months?’,15 the 2309 responders (n=1136 girls) were divided into two groups. The adolescents who responded positively to the question were defined as having exercise-induced dyspnoea (n=330), while those who responded negatively were defined as controls (n=1979). There were more girls than boys in the group reporting exercise-induced dyspnoea (58% vs 42%, respectively; p<0.001).
Clinical phase
After stratifying for gender according to the results of the screening phase (female:male ratio 3:2), the adolescents with exercise-induced dyspnoea and the controls were randomly sequenced using computer randomisation. A total of 199 adolescents with exercise-induced dyspnoea and 123 controls were randomly selected and invited to participate in the study. The first 103 adolescents with exercise-induced dyspnoea and the first 47 healthy controls who agreed to participate were recruited for the clinical phase of the study which took place between March 2012 and June 2013. The exclusion criteria were pulmonary disease apart from asthma, cardiac co-morbidity or a functional disability that resulted in inability to perform exercise tests.
Pre-test visit
Age, sex, height and weight were recorded and body mass index (BMI) (kg/m2) was calculated. The use of inhaled corticosteroids, β2-agonists and leukotriene receptor antagonists during the last 3 months was recorded. The participants were instructed on how to prepare for the two exercise tests, which were conducted on separate days.
EIB test
The EIB test was performed on average a median (IQR) of 12 (6–19) days after the pre-test visit. The investigator (HJ) who collected the data was blinded to whether the participants belonged to the exercise-induced dyspnoea group or the control group.
Participants were instructed to cease short-acting β2-agonists 8 h before the test, long-acting β2-agonists 24 h before the test and leukotriene receptor antagonists 72 h before the test. They were also instructed not to use inhaled steroids on the day of the test and to avoid vigorous exercise, heavy meals, caffeine-containing food or beverages and nicotine for 4 h before the test.
Baseline spirometry was performed according to ATS/European Respiratory Society (ERS) guidelines16 and the pre-challenge forced expiratory volume in 1 s (FEV1) was documented as the best FEV1 of three measurements (CardioPerfect dynamic spirometry; Welch Allyn, Höganäs, Sweden). During the standardised 6 min treadmill exercise test (Marquette Series 2000 Treadmill; GE, Waukesha, Wisconsin, USA), the subject wore a nose clip and breathed through a tube (Aiolos Asthmatest; Aiolos Medical, Karlstad, Sweden) connected to a central gas container with dry air (H2O <5 mg/L, 18–22°C). Heart rate was continuously monitored using a heart monitor (CASE Exercise Testing System; GE Medical Systems, Milwaukee, Wisconsin, USA). The end-point was to increase cardiac frequency to 90% of the predicted maximum ((208−(0.7×age))×0.9))17 within the first 1.5 min and maintain this level throughout the 6 min test by adjusting the treadmill speed and slope. FEV1 was measured 5, 10, 15 and 30 min after the test. The best FEV1 value of two measurements at each time point was documented. EIB was defined as a decrease of ≥10% in FEV1 from baseline.4
Continuous laryngoscopy exercise test
The continuous laryngoscopy exercise (CLE) test was performed a median (IQR) of 38 (22–66) days after the EIB test. The investigator (KN) was blinded to whether the participants belonged to the exercise-induced dyspnoea group or the control group as well as to the EIB test result.
Participants were informed that there were no restrictions regarding asthma medication, that is, those who usually took asthma medication before physical exercise should also do so before the CLE test. Participants were instructed to avoid vigorous exercise on the day of the test and heavy meals for 4 h before the test.
The CLE test was performed according to the method described by Heimdal et al12 with minor modifications. Before the test, naphazoline-lidocaine was sprayed twice into each nostril to achieve local anaesthesia and dilatation. The participant warmed up on the treadmill at 10 km/h for 2 min (Master Fitness T590; Master Fitness, Guangzhou, China). The treadmill was stopped and a fibre optic laryngoscope (Olympus ENF-P3; Olympus, Southend-on-Sea, UK) was inserted in one nostril. The tip of the laryngoscope was placed just above the epiglottis providing a detailed view of the larynx. The laryngoscope was fastened in a specially designed helmet so that it stayed in position, and was connected to a camera (Ubicam; Sopro Imaging, La Ciotat, France). The participant was equipped with a heart rate monitor consisting of a chest strap and wrist watch (T31; Polar, Oulu, Finland).
The participant started running at a speed of 9 km/h with no inclination, and the speed was increased by 1 km/h every minute until it reached 13 km/h. Thereafter, the inclination was increased by 2% every minute. The participants were instructed to run until breathing problems forced them to stop, or, if no breathing problems occurred, until exhaustion. For the test to be considered successful, the heart rate had to reach ≥90% of predicted maximum.17 The larynx was filmed continuously during the test and the larynx findings were graded immediately after the test according to the criteria described by Maat et al.13 An obstruction grade of 2 or more at either the supraglottic or the glottic level, or both, was considered to be a positive response and was defined as EILO.13
Dropouts from the study
Four subjects with exercise-induced dyspnoea declined participation in the exercise tests, leaving 146 subjects (99 with exercise-induced dyspnoea and 47 healthy controls) who performed the EIB test. For the CLE test, another 19 subjects (14 subjects from the group with exercise-induced dyspnoea) declined participation and two subjects were excluded due to fainting during the CLE test and equipment failure (figure 1).
Statistical analysis
The data were analysed using Statistical Package for Social Science (SPSS) software, V.21 (SPSS, Chicago, Illinois, USA). Anthropometric data, lung function, asthma medication and exercise test results were summarised as the means, SDs and minimum and maximum for continuous variables, and as numbers and percentages for categorical variables. Age, BMI and FEV1 were compared between subject groups using unpaired Student's t tests. For all categorical variables, a cross-tabulation versus subject groups was performed and subject groups were compared using Fisher's exact test. The results were considered to be statistically significant at p<0.05.
The EIB and EILO prevalence estimates were based on the assumptions that the population strata with and without exercise-induced dyspnoea differed. Assuming that there was no selection bias, the dyspnoea stratum with exercise-induced dyspnoea was calculated from the rate of exercise-induced dyspnoea among responders multiplied by the population size, and the stratum without exercise-induced dyspnoea was assumed to represent the rest of the population. The adolescents who underwent the EIB and CLE tests were assumed to be random samples from the two strata. The prevalence estimate and 95% CI were calculated according to the theory for stratified samples.18
Results
Of the 150 subjects who were referred for assessment, 103 had exercise-induced dyspnoea and 47 were controls. Group characteristics are presented in table 1. In the group with exercise-induced dyspnoea, more adolescents reported ever having been diagnosed with asthma by a physician and they had used more asthma medication compared to controls.
Population and group characteristics
Prevalence of EIB in the total population
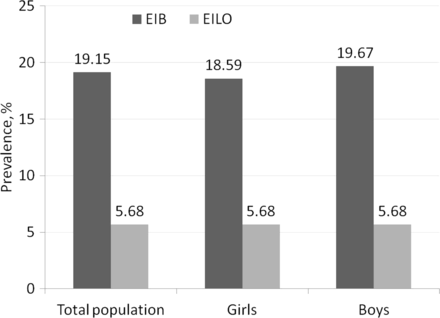
EIB was present in 42 (42.4%) adolescents in the exercise-induced dyspnoea group and in seven (14.9%) adolescents in the control group (table 1). The estimated prevalence of EIB in the population of 3838 adolescents was 19.2% (95% CI 9.9% to 28.4%), 18.6% in girls and 19.7% in boys (p=0.91) (figure 2).
Prevalence (%) of exercise-induced bronchoconstriction (EIB) and exercise-induced laryngeal obstruction (EILO) in the total population and in girls and boys.
Prevalence of EILO in the total population
EILO was present in nine (10.8%) adolescents in the exercise-induced dyspnoea group and in two (4.8%) adolescents in the control group (table 1). The estimated prevalence of EILO in the population was 5.7% (95% CI 0.01% to 11.4%) with no gender differences (p>0.99) (figure 2).
Adolescents with exercise-induced dyspnoea without EIB or EILO
Of the adolescents with exercise-induced dyspnoea, 49.4% had neither EIB nor EILO (figure 3). Significantly more boys than girls did not have EIB or EILO (64.7% vs 38.8%; p=0.026) (table 2 and figure 4). There were no significant difference in BMI, lung function, ever asthma or medication between those with and without a positive EIB and/or EILO test (table 2). Regarding self-reported exercise induced symptoms there were no differences between participants with exercise-induced dyspnea that had or did not have EIB or EILO (see online supplementary table).
Characteristics of subjects with exercise-induced dyspnoea without EIB and EILO versus subjects with exercise-induced dyspnoea with EIB and/or EILO
Exercise-induced bronchoconstriction(EIB) and exercise-induced laryngeal obstruction (EILO) in adolescents reporting exercise-induced dyspnoea.
Exercise-induced bronchoconstriction (EIB) and exercise-induced laryngeal obstruction (EILO) in boys and girls reporting exercise-induced dyspnoea.
Dropouts from the study
The adolescents with exercise-induced dyspnoea who declined to participate in the clinical phase of the study (n=93) did not differ from the group of participating adolescents with exercise-induced dyspnoea (n=103) regarding age, gender or BMI; however, a larger proportion of participating adolescents reported ever asthma (48% vs 32%; p=0.028). Similarly, a larger proportion of participating controls (n=47) compared to controls who declined to participate (n=76) reported ever asthma (21% vs 4%; p=0.002); however, the groups did not differ regarding age, gender or BMI.
Discussion
The present cross-sectional study demonstrated that EIB and EILO, assessed using standardised exercise tests, are common in a general population of adolescents. The estimated prevalence of EIB was 19.2% and the estimated prevalence of EILO was 5.7%, with no gender differences. In subjects with exercise-induced dyspnoea, EILO alone and EIB in combination with EILO were found to a similar extent. In approximately half of the subjects with exercise-induced dyspnoea, neither EIB nor EILO could be demonstrated and the unexplained self-reported exercise-induced dyspnoea was more common among boys than girls.
This prospective study from a general population sample is the first to present data regarding EIB, EILO and co-occurrence of both together. This study is also the first to investigate the prevalence of EIB, in a general population, using inhalation of dry air during the standardised exercise challenge test in accordance with recent clinical guidelines from the ATS.4
In the present study the estimated prevalence of EIB was 19.2%, which is higher than previously reported. The prevalence of EIB among 10–12-year-old children in Algeria was 16.1% when the 6 min free-running test was used and EIB was defined as a decrease of ≥15% in peak expiratory flow rate.10 Using the same exercise protocol for investigating 13–14-year-old adolescents, Busquets et al9 found a prevalence of 11%, while De Baets et al7 found a prevalence of 7.4% among primary school children. Hallstrand et al8 found an EIB prevalence of 9.4% when studying 256 adolescents; they used the 6 min free-running test but defined EIB as a decrease in FEV1 of ≥10%. Lødrup Carlsen et al19 reported an EIB prevalence of 8.6% (defined as a decrease in FEV1 of ≥10%) when 616 children (mean age 10.9 years) were exercise tested in a laboratory setting. The fact that in the present study, unlike in other studies, the subjects breathed dry air with a nose clip in place during the exercise test might have increased the sensitivity of the test, thereby increasing the prevalence. It is also possible that differences in climate conditions may partly account for differences in the reported prevalence of EIB in different geographical areas. EILO was not uncommon in our population and its prevalence was estimated to be 5.7%. The only previous prospective study investigating a general population (aged 14–24 years; mean age, 18.6 years) found a prevalence of 7.6%.14 However, the authors of that study concluded that the result might have been influenced by the low number of participants assessed for EILO, as only 98 (17.6%) of 556 invited participants underwent the CLE test.
We did not find any gender differences in the estimated prevalence of EIB. However, data from previous studies are inconsistent. De Baets et al7 found an EIB prevalence of 8.5% in girls and 6.4% in boys, while Busquets et al9 also reported a higher prevalence of EIB among girls (13%) than boys (9%). In contrast, other studies did not reveal any gender differences.10 ,20
We investigated 13–15-year-old adolescents and found that the estimated prevalence of EILO was similar (5.7%) in girls and in boys. Christensen et al reported a higher prevalence of EILO in females (OR females/males 1.61),14 while several case reports on EILO and vocal cord dysfunction found the majority of patients were female.21–24 One plausible reason why more females had EILO in the study by Christensen et al could be because the population was older (14–24 years). By the age of 16–17 years, the male larynx has reached adult size, most likely reducing the problem for male individuals. In the present study, several males had gone through puberty, while other males were still prepubertal and had the narrow larynx of a child, which likely explains the lack of difference between genders.
Almost half of the participants with EILO also had EIB, so EILO and EIB might be related. This notion is partly supported by the findings that expiratory glottic constriction sometimes occurs during induced bronchial asthma.25 However, in our study none of the participants with both EILO and EIB had glottic closure. We therefore find it more likely that EILO is independent of EIB.
The fact that EIB and EILO are not mutually exclusive is important from a treatment perspective: if tested positive and successfully treated for EIB, an adolescent with both EIB and EILO may still experience breathing problems in conjunction with exercise. Thus, exercise testing for both EIB and EILO is a precondition for identifying subjects with both causes of transient airway narrowing.
A substantial proportion (49%) of the participants reporting exercise-induced dyspnoea did not test positive for EIB or EILO. This finding is in agreement with previous studies. Abu-Hasan et al26 retrospectively reviewed exercise tests in 117 patients with exercise-induced dyspnoea (mean age, 14 years) and concluded that 52% did not have cardiopulmonary abnormalities. One reason for exercise-induced dyspnoea is poor physical condition.27 In addition to being unfit, exercise-induced respiratory symptoms can have other causes such as restrictive abnormalities, primary hyperventilation, cardiac disease and congenital malformations.28 ,29 Further studies are needed in order to explain the underlying reason for exercise-induced dyspnoea in adolescents who have neither EIB nor EILO.
A larger proportion of the participating adolescents reported ever asthma compared to those who had declined participation. This result may have led to an overestimation of the prevalence estimates of EIB in the total population. Moreover, 25 participants in the clinical phase of the study did not complete both exercise tests; however, this group did not differ from the group completing both tests regarding baseline characteristics (data not shown). These dropouts are therefore unlikely to affect the prevalence estimate of EILO.
In conclusion, EIB is common among adolescents experiencing exercise-induced respiratory symptoms. EILO is also not uncommon in the general population and can be an important differential diagnosis in adolescents experiencing breathing problems in conjunction with exercise. EIB and EILO are not mutually exclusive and can co-exist; the diagnosis should be confirmed using standardised exercise tests.
Acknowledgments
The authors thank participating adolescents and their guardians. The authors thank H. Hedenström for contributing to the study design and P. Kalm-Stephens and K. Nisser for technical assistance.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online table
Footnotes
-
*Henrik Johansson and Katarina Norlander have shared first authorship on this paper.
-
Contributors HJ and KN contributed to study design, data collection, analysis and the first draft of the manuscript; LB contributed to study design, using prevalence estimates, statistical analysis and review of the manuscript; CJ, AM, LNordv and ME contributed to study design, analysis and review of the manuscript; LNorda contributed to study design, data collection, analysis and review of the manuscript; HJ, KN and ME had full access to all of the data in the study and were responsible for the integrity of the data and the accuracy of the data analysis.
-
Funding This study was sponsored by the Swedish Heart Lung Foundation (grant number 20110179), the Signhild Engqvist Foundation, the Bror Hjerpstedt Foundation and the Gillbergska Foundation.
-
Competing interests None.
-
Ethics approval The ethical review board in Uppsala, Sweden provided ethics approval for this study (Dnr 2011/413).
-
Provenance and peer review Not commissioned; externally peer reviewed.