Article Text
Abstract
Neural respiratory drive (NRD), as reflected by change in parasternal muscle electromyogram (EMGpara), predicts clinical deterioration and safe discharge in patients admitted to hospital with an acute exacerbation of COPD (AECOPD). The clinical utility of NRD to predict the long-term outcome of patients following hospital admission with an AECOPD is unknown. We undertook a post hoc analysis of a previously published prospective observational cohort study measuring NRD in 120 patients with AECOPD. Sixty-nine (57.5%) patients died during follow-up (median 3.6 years). Respiratory failure was the most common cause of death (n=29; 42%). In multivariate analysis, factors independently associated with an increased mortality included NRD (HR 2.14, 95% CI 1.29 to 3.54, p=0.003), age (HR 2.03, 95% CI 1.23 to 3.34, p=0.006), PaCO2 at admission (HR 1.83, 95% CI 1.06 to 3.06, p=0.022) and long-term oxygen use (HR 2.98, 95% CI 1.47 to 6.03, p=0.002). NRD at hospital discharge could be measured in order to assess efficacy of interventions targeted to optimise COPD and reduce mortality following an AECOPD.
Original clinicaltrial.gov number: NCT01361451
- copd exacerbations
- respiratory muscles
- respiratory measurement
Statistics from Altmetric.com
Introduction
Following a severe acute exacerbation of COPD, patients are at high risk of readmission and death.1 2 Neural respiratory drive (NRD) can be estimated using parasternal muscle electromyogram (EMGpara). EMGpara is a non-invasive physiological measurement which has demonstrated utility in determining the trajectory of recovery during hospital admission as well as predicting readmission following a hospital admission.3 Indeed, Suh et al showed that NRD can predict early 14-day readmission in 120 unselected patients with acute exacerbations of COPD.4 Although there are composite measures that predict long-term outcome based on disease severity in stable COPD,5 6 there are few factors predicting the long-term outcome at the end of a severe exacerbation of COPD. The aim of the current study was to assess if NRD at hospital discharge following an admission due to an exacerbation of COPD could predict long-term mortality.
Methods
The original study was registered as an observational cohort study (NCT01361451). The study was conducted between January 2011 and September 2013. Full details of the protocol are available in the cohort manuscript.4 In brief, consecutive patients admitted to a UK urban teaching hospital with acute exacerbations of COPD were recruited and had daily measurements of NRD, using EMGpara, from admission until discharge.4 In May 2017, we collected data from electronic medical records to assess the mortality status of all recruited patients. Cause of death was obtained from the medical cause of death certificate. EMG data are expressed as EMGpara (which corresponds to the measured EMGpara during tidal breathing), EMGpara%max (which corresponds to the ratio between EMGpara during tidal breathing and EMGpara during a maximal sniff manoeuvre) and NRD index (NRDI) (which corresponds to EMGpara%max*RR).
Data were assessed for normality using the Shapiro-Wilk test. Results are expressed as number and percentages, means and SD when normally distributed or medians and IQR when not normally distributed. Comparisons were performed using the t-test for normally distributed continuous variables and a Mann-Whitney U test for non-normally distributed continuous variables. Survival data were analysed using Kaplan-Meier method and log-rank test. Prognostic factors were identified using a multivariate Cox model. Prognostic factors with a p value <0.1 in univariate analyses were included in the multivariate Cox model. EMGpara%max was the only variable included in the model given its collinearity with NRDI. For the Cox model, all continuous variables were divided into two groups based on their median. For admission PaCO2, the study population was divided into two groups: ≥6 or <6 kPa. Receiver-operator characteristic (ROC) analyses were used to assess performance of predictors of 5 years mortality. A predictive model was produced using logistic regression with variables from the Cox model. All tests were two-sided with the level of significance set at 0.05. Analyses were performed using GraphPad Prism V.6 for Mac OS X (GraphPad Software, La Jolla, California, USA) and IBM SPSS Statistics V.20.0 (IBM Corp, Armonk, New York, USA).
Results
Of the 120 patients involved in the clinical trial, 69 (57.5%) had died by 1 May 2017, mean duration of follow-up was 3.4±1.8 years. Patients’ clinical and physiological characteristics obtained during hospital admission are reported in table 1. During inpatient stay, eight (6%) received acute non-invasive ventilation. Medical cause of death was reported as respiratory failure for 29 (42.0%) patients, cardiac failure for five (7.2%), cancer for five (7.2%) and from other cause for two (2.9%). Cause of death could not be verified in 26 (37.7%) patients.
Population characteristics
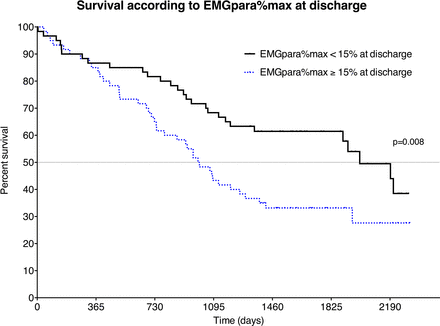
Regardless of the cause of death, an EMGpara%max at discharge ≥15% was associated with a worse prognosis with a median survival of 998 days versus 2002 days when compared with an EMGpara%max at discharge <15% (HR 1.89, 95% CI 1.19 to 3.08, p=0.009, log rank) (figure 1). Other prognostic factors in the univariate analysis are reported in the online supplement etable 1. A post hoc analysis of respiratory-specific mortality indicated a stronger association with long-term outcomes with an EMGpara%max at discharge ≥15% being associated with a threefold risk of respiratory-specific mortality when compared with an EMGpara%max <15% (HR 3.18, 95% CI 1.52 to 6.46, p=0.002, log rank, online supplement efigure 1).
Supplemental material
Survival according to EMGpara%max at discharge (continuous line: patients with EMGpara%max <15% at discharge, dot: patients with EMGpara%max ≥15% at discharge) (p=0.008, log rank). EMGpara, parasternal muscle electromyogram.
In the multivariate analysis, factors associated with a poor prognosis were an EMGpara%max at discharge ≥15% (HR 2.14, 95% CI 1.29 to 3.54, p=0.003), an age ≥71 years (HR 2.03, 95% CI 1.23 to 3.34, p=0.006), a PaCO2 at admission ≥6 kPa (HR 1.83, 95% CI 1.06 to 3.06, p=0.022) and previous long-term oxygen use (HR 2.98, 95% CI 1.47 to 6.03, p=0.002). In the multivariate analysis, FEV1 at discharge and hospital admission frequency were not associated with long-term outcome (p=0.606 and 0.720, respectively).
ROC analysis for the prediction of 5 years mortality gave an area under the curve of 0.649, 0.626, 0.701 for EMGpara%max, age and admission PaCO2, respectively (p=0.02, 0.006, <0.001, respectively). An individual risk score of all significant factors associated with poor prognosis was produced to predict 5-year mortality and provided an area under the curve of 0.747 (p<0.001) (online supplement etable 2 and online supplement efigure 2). The individual risk score performed better than the isolated predictors (EMGpara%max, p<0.001; age, p<0.020; admission PaCO2, p=0.201). The regression model provided an individual risk score correctly classifying 74% of patients (online supplement etable 3).
Discussion
This post hoc analysis has demonstrated that NRD, as reflected by EMGpara, is an independent predictor of long-term mortality in patients following an admission for an acute exacerbation of COPD. NRD, as reflected by EMGpara%max, at admission was similar between patients who died and those who remained alive at follow-up, as was early readmission within 28 days and 12-month mortality, indicating that the difference was likely not due to the severity of the index exacerbation. However, those who remained alive appear to have responded more favourably to treatment during the acute exacerbation with a reduction in EMGpara, EMGpara%max and NRDI indicating a favourable effect on the load–capacity–drive relationship of the respiratory system. Conversely, patients who died during follow-up demonstrated smaller changes in measures of NRD and therefore high levels at discharge, indicating either less reversible disease or more severe baseline COPD. This lack of improvement associated with a higher NRD at discharge highlights the severity of their respiratory disease more accurately than traditional markers such as FEV1, the number of admissions for acute exacerbation of COPD or symptom-related questionnaires. The importance of an imbalance in the load–capacity–drive relationship of the respiratory system is highlighted by the fact that the principal cause of death was from respiratory failure rather than cardiovascular causes as is common in mild COPD.7
In our cohort, factors associated with poor survival were prescription of long-term oxygen therapy, age, admission PaCO2 and EMGpara%max. Interestingly, the last two parameters can be treated with non-invasive ventilation providing a possible physiological rational for recently demonstrated benefits of this therapy.8 9 Our findings are consistent with those of Esteban10 and colleagues who demonstrated a link between 1-year survival and patient characteristics (age), clinical severity of COPD (presence of hypercapnia) and comorbidities in a large multicentre study. These findings demonstrate a potential role for NRD in the risk stratification of patients following a hospital admission secondary to an acute exacerbation of COPD. Future work will need to focus on strategies to reduce NRD to evaluate whether these can alter disease progression, risk of re-exacerbation and hospitalisation and long-term mortality.
Conclusion
In addition to the short-term value of using NRD to predict safe hospital discharge following a severe acute exacerbation of COPD, NRD at hospital discharge could be measured in order to assess efficacy of interventions targeted to optimise COPD and to reduce mortality following an acute exacerbation of COPD.
Acknowledgments
Authors would like to thank Jennifer Owusu-Afriyie and Laura Allum for their help in data collection.
Footnotes
NH and PBM are joint senior authors.
Contributors MP, ESS, NH and PBM contributed to the conception of the work, analysis, interpretation of data and drafting of the work. LM, RB, GA, EL, GK, NH and PBM critically revised the draft. All authors contributed to the acquisition of data.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests MP reports grants from B&D Electromedical, during the conduct of the study; personal fees from Resmed and Philips-Respironivd, grants and non-financial support from Fisher & Paykel, non-financial support from MSD, non-financial support from Asten, grants from ADIR Association, outside the submitted work. EL reports personal fees and non-financial support from ASTEN, outside the submitted work. GK, RD, LM and GA have nothing to disclose. E-SS Suh reports grants from Philips Research, outside the submitted work. NH Pulmonary Research Advisory Board for Philips and the funds for this is given to Guy’s and St Thomas’ NHS Foundation Trust. My research group has received unrestricted grants (managed by Guy’s and St Thomas’ Foundation Trust) from Philips-Respironics, Philips, Resmed, Fisher-Paykel and B&D Electromedical. Philips and Philips-Respironics are contributing to the development of the MYOTRACE technology. PBM reports grants and personal fees from Philips-Respironics, grants and personal fees from Resmed, grants and personal fees from B&D electromedical, outside the submitted work.
Patient consent for publication Not required.
Ethics approval The original study was approved by the London-Bentham Research Ethics Committee. Post hoc analysis of the original study data was approved by Guy’s & St Thomas’ Research Ethics Committee (16/70/1773).
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement There are no additional unpublished data from the study.
Linked Articles
- Airwaves